Reference no: EM13671311
Question 1
Rachel Green, the owner-manager of a small business, had carefully monitored her cash position over the past financial year, and was pleased to note at the end of the year that the cash position was strong and had shown a healthy 50% increase over the year. When presented with the income statement for the year, she was dismayed to note that the profit earned in the last year had deteriorated significantly and had become a loss for the current financial year. In her anger, she accuses you of having made errors in the accounting records since "such a silly situation could not possible exist". Draft a response to Rachel.
Question 2
After calculating the current ratio of an entity and finding that the ratio's value was 5:1, a student analyst decided that the company was in a sound position for paying all its liabilities including all non-current liabilities. Discuss the shortcomings of making such a conclusion. Also outline some of the disadvantages of using ratio analysis only to evaluate a company's performance.
Question 3
In analysing the financial statements of an entity, the following ratios were calculated:
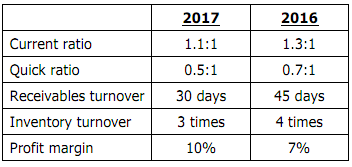
What conclusion(s) can be made about the entity based on the above ratios?
Question 4
Below are the comparative statements of financial position of Soccer ltd:
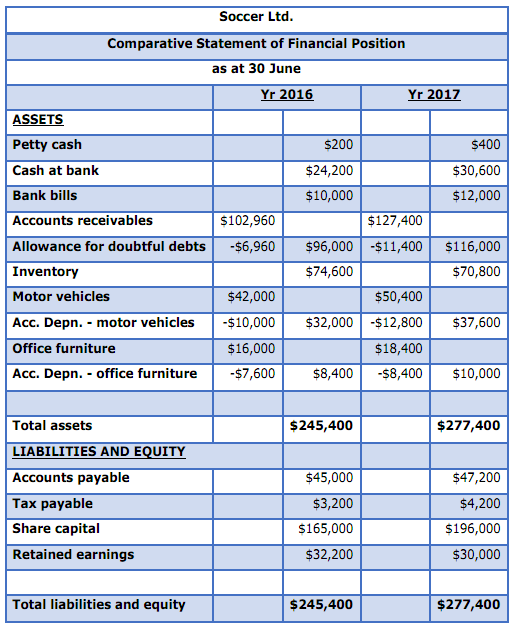
Additional information:
1. Income statement details are: sales revenue $750,000, cost of sales $603,000, expenses $116,360 (excludes depreciation and carrying amount of vehicle sold), bad debt expense $14,440 and tax expense $4,200
2. A dividend was paid using the year
3. A vehicle that cost $5,600 originally was sold during the year for $3,000. The vehicle had been depreciated by $3,200 at date of sale.
4. The company pays income tax in one instalment. The single instalment of $3,200 due by 21 Oct 2016 was paid.
Required
Prepare a statement of cash flows for the year ended 30 June 2017 in accordance with the direct method.
Question 5
The following information has been extracted from the financial statement and notes of Reef Ltd:
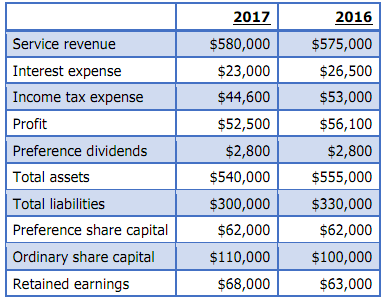
Required: Evaluate the company's profitability and financial stability by calculating and analysing the relevant ratio(s).
|
Define the length of a side of the unit cell for compounds
: Given the subsequent data for the length of a side of the unit cell for compounds that crystallize in the rock salt structure, find out the cation radii: MgSe (545pm), CaSe (591 pm), SrSe(623pm), BaSe (662 pm). It is known that the Se2-ions are in..
|
|
Explain the short run shut down rule
: Calculate the firm's profit or loss. Is the firm making a profit or a loss and explain the Short Run Shut Down Rule. Should this firm shut down? Please explain.
|
|
State compounds that crystallize in the rock salt structure
: Given the subsequent data for the length of a side of the unit cell for compounds that crystallize in the rock salt structure, find out the cation radii: MgSe (545pm), CaSe (591 pm), SrSe(623pm), BaSe (662 pm). It is known that the Se2-ions are in..
|
|
Define which would give a basic solution p4o10
: Which of the subsequent would an acidic solution when they react with water? Define which would give a basic solution? (a)P4O10, (b) K2O, (c)SeO3, (d) Cl2O7
|
|
Evaluate the companys profitability and financial stability
: Prepare a statement of cash flows for the year ended 30 June 2017 in accordance with the direct method - evaluate the company's profitability and financial stability by calculating and analysing the relevant ratio(s).
|
|
Compute the radius of cesium ion in cscl
: Compute the radius of cesium ion in CsCl if the density is 3.97g/cm3 as well as it is assumed that the ions touch through the body diagonal the unit cell
|
|
Define the valence band plus the conduction band
: In metallic magnesium what fraction of the molecular orbits restricted in the valence band plus the conduction band are in use by electrons
|
|
Explain why are their molecule geometries different
: The molecules methane (CH4) as well as ammonia (NH3) both has four regions of high electron density around their central atoms, and yet these two molecules have different molecular geometries. Explain why are their molecule geometries different
|
|
How many total electrons does each of the cations have
: Consider the five individual ions two cations as well as three anions that come together to form the compound ferric oxide. How many total electrons does each of the cations have
|