Reference no: EM132421110
Assignment Questions -
Q1. Draw the following molecules and indicate if they are chiral and circle the tetrahedral stereo-centers.
a. Methylcylcohexane
b. 2-Methylcyclohexanone
c. 5-Bromodecane
d. 2-amino-3-hydroxybutanoic acid
e. 4-Methyl-1-hexene
Q2. Label the following as aldohexose, aldopentose, ketopentose, ketohexose, etc
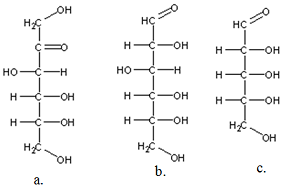
Q3. Draw all the stereo-isomers for 2a. as enantiomeric pairs.
Q4. Given the Fisher projection of the carbohydrates (shown below), draw both the alpha and beta anomers for the corresponding Haworth projections.
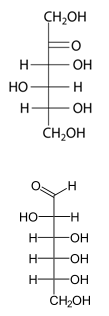
Q5. Draw the corresponding Fisher projection and indicate if the compound is D or L. (Hint assume the -OH group at carbon 5 on the fisher projection forms the bond with carbon 1 observed on the Haworth projection).
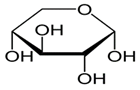
Q6. For the following carbohydrate molecules, determine if they are α or β (and circle the OH group in question).
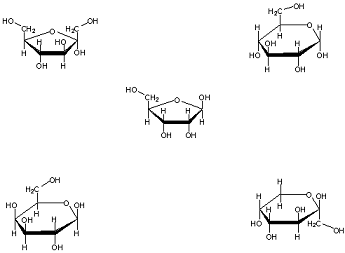
Q7. Consider the following polysaccharide:
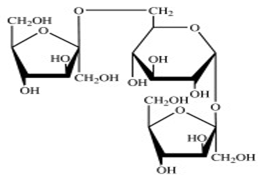
a) What is the linkage between each sugar unit?
b) Hydrolysis is the process of converting polysaccharides into its monosaccharides. Draw the products upon hydrolysis?
Q8. Consider the following polysaccharide:
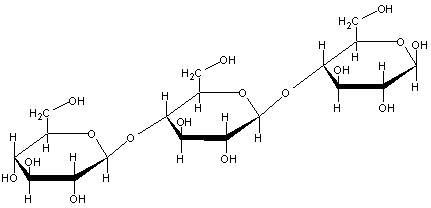
a) What is the linkage between each sugar unit?
b) Draw the products upon hydrolysis?
|
If the lengths of the sides of a4bc is 3 x+3 and 9 which
: If the lengths of the sides of A4BC is 3, x+3, and 9, which of the following could be the value of x?
|
|
Explain how the cost principle applies to plant assets
: Discuss the three methods for calculating depreciation on plant assets (straight line, declining balance, and units of activity).
|
|
What are the seven media literacy skills
: What characteristics did they display that clued you into their listening styles? How did they influence that day's communication?
|
|
Discuss the reason for the inmate lawsuit
: Please pick an appellate case dealing with each of these amendments (3 cases total). Discuss the reason for the inmate lawsuit.
|
|
Draw the corresponding fisher projection
: Draw the corresponding Fisher projection and indicate if the compound is D or L. Draw all the stereo-isomers for 2a. as enantiomeric pairs
|
|
What are the various types of control
: What are the various types of control? What is performance evaluation and methods of corrective action? What is the definition of productivity?
|
|
How did the course affect your views on technology
: How did this course affect your views on technology? Do you feel more or less optimistic about your personal security? How will the knowledge gained affect.
|
|
Calculate the first year net earnings
: In its first year of operations, Blossom Company recognized $33,400 in service revenue, $7,700 of which was on account and still outstanding at year-end.
|
|
Which country is contradictory to governmental structure
: If you were a world history professor, which country that you read about this week is the most contradictory to the governmental structure we have.
|