Reference no: EM132518878
Question - Consider SF3-. Recall from MT1 question 3e that the molecule adopts a T-shape and belongs to the C2v point group.
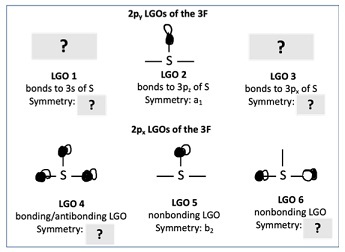
a) For question, making the following assumptions: the 2s LGOs of the 3F are too low in energy to interact with the sulfur atomic orbitals and the 2pz LGOs of the 3F have poor overlap with the sulfur atomic orbitals. Therefore, the molecular orbitals are dominated by interactions between the central sulfur atomic orbitals and the 2py and 2px LGOs of the 3F. Consider the incomplete figure below of the 2py and 2px LGOs of the 3F. Fill in the missing pieces (draw LGO 1 and 3, provide symmetries of LGO 1, 3, 4, and 6.
(Hint for LGO 6: you can use the projection method to determine the symmetry of LGO 4; LGO 6 is what remains from your irreducible representation.)
b) Draw the complete MO diagram of SF3- (including electrons and symmetry labels of all MOs). Use dotted lines from AOs to MOs to indicate primary interactions. In addition to the assumptions stated in part b (the 2s and 2pz LGOs will not interact with the central S atomic orbitals), make the following assumptions:
Due to overlap, LGO 1 will primarily interact with the 3s orbital of sulfur (and minimally with the 3pz orbital of sulfur).
Due to overlap, LGO 2 will primarily interact with the 3pz orbital of sulfur (and minimally with the 3s orbital of sulfur).
LGO 5 shares the same symmetry as either LGO 4 or LGO 6. Due to poor overlap relative to one of these, LGO 5 will effectively be nonbonding.