Reference no: EM13278381
1. (i) Draw Newman projections for the 6 rotational isomers of 3-methylpentane visualizing down the C2-C3 bond. (i) Draw the energy diagram for a 360 deg rotation about this bond, indicating the relative energies of the 6 species.
2. (i) Draw the two chair conformations for trans-2,3-dimethylcyclohexane. (ii) Draw the two chair conformations for cis-2,3-dimethylcyclohexane. (iii) Which isomer is more stable and why?
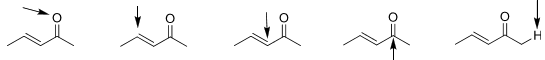
3. For the following, determine if the atoms (indicated by the arrow) are electrophilic, nucleophilic, or neither.
4. Propose reasonable mechanism for the following transformation using curved arrow notation.
5. Provide the major product for the following reactions. Use curved arrow notation to indicate the flow of electrons.
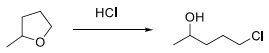
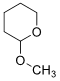
6. (i) Draw a mechanism using curved arrow notation for the acid-catalyzed cleavage (hydrolysis) of the following molecule.
(ii) Synthesize the following molecule using the appropriate carbonyl compound.
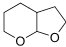
7. The beta-blocker atenolol is commonly prescribed for high blood pressure and is used to slow the heart rate of patients at risk for "a-fib". The molecular structure is shown below.
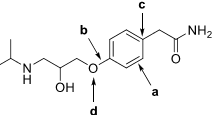
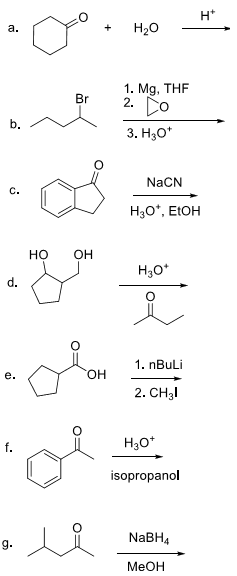
a. What is the molecular formula?
b. Circle and name all functional groups present.
c. Provide bond descriptions for bonds a and b.
d. Give the hybridization of carbon c and oxygen d.
e. Explain how the hybridization of carbon c occurs.
f. Draw the VB representation (orbital overlap) of the bonds shown below.
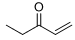
8. For the following acid base reactions, (a) write the expected products, (b) label all acids and bases and their conjugates, (c) estimate the pKa values, and (c) predict the direction of the equilibrium.
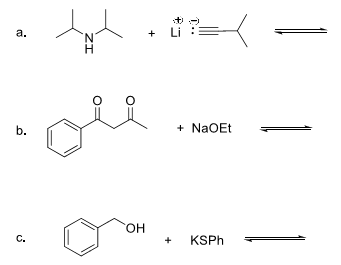
9. In the following chiral pharmaceuticals, indicate ALL stereocenters with an asterisk (*) and assign the absolute configuration (R or S).
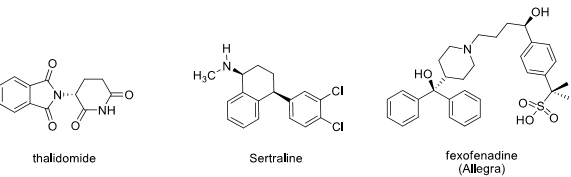
10. Explain the following observation. Be sure to include resonance structures in your discussion and use structures where appropriate.
|
Calculate the impulse exerted on the tackler
: A 85.0 kg fullback is running at 3.86 m/s to the east and is stopped in 0.800 s by a head-on tackle by a tackler running due west. Calculate the impulse exerted on the tackler
|
|
Define the formation of an insoluble precipitate
: pairs of soluble solutions can be mixed. In some cases, this leads to the formation of an insoluble precipitate. Decide, in each case, whether or not an insoluble precipitate is formed.
|
|
What is current in circuit a long time after switch is close
: A circuit contains a known resistance of 2.4 kO, and an unknown inductance. a) When a 12-V battery is connected, what is the current in the circuit a long time after the switch is closed
|
|
What is the mass of the second car
: A 8500 kg boxcar traveling at 16.8 m/s strikes a second boxcar at rest. What is the mass of the second car
|
|
Draw newman projections
: Draw Newman projections for the 6 rotational isomers of 3-methylpentane visualizing and draw the two chair conformations for trans-2,3-dimethyl cyclohexane
|
|
Explain p-aminophenol what was the limiting reageant
: If you used 0.165mL of acetic anhydride, and 0.167g of p-aminophenol what was the limiting reageant? How many moles of the non-limiting reageant remained after the reaction was complete
|
|
What is the wave speed for this string
: A string is stretched between fixed supports separated by 73.0 cm. It is observed to have resonant frequencies of 390, What is the wave speed for this string
|
|
Design a four-bit shift register using a barrel shifter
: A four-bit barrel shifter is a combinational circuit with four data inputs, two control inputs, and two data outputs. It allows the data inputs to be shifted onto the outputs by 0, 1, 2, or 3 bit positions, with the rightmost bits wrapping around ..
|
|
Explain thermochemical equation for the decomposition
: Consider the following balanced thermochemical equation for the decomposition of the mineral magnesite: MgCO3(s) => MgO(s) + CO2(g) ?Hrxn = 117.3 kJ What is ?H in kJ when 41.56 g of CO2 reacts with excess MgO
|