Reference no: EM13839306
1. The hormone estrogen is produced in the ovaries of females and elsewhere in the body in men and post-menopausal women, and it is also administered in estrogen replacement therapy, a common treatment for women who have undergone a hysterectomy. Unfortunately, it also binds to estrogen receptors in breast tissue and can activate cells, in effect blocking the receptor from access to estrogen and inhibiting the growth of breast-cancer cells.
Tamoxifen is administered in tablet form. In the manufacturing process, a finely ground powder contains tamoxifen (tam) and two inactive fillers-lactose monohydrate (lac) and corn starch (cs). The powder is mixed with a second stream containing water and suspended solid particles of polyvinylpymo-lidone (pvp) binder, which keeps the tablets from easily crumbling. The slurry leaving the mixer goes to a dryer, in which 94.2% of the water fed to the process is vaporized. The wet powder leaving the dryer contains 8.80 wt% tam, 66.8% lac, 21.4% cs, 2.00%pvp, and 1.00% water. After some additional processing, the powder is molded into tablets. TO produce a hundred thousand tablets, 17.13 kg of wet powder is required.
(a) Taking a basis of 100,000 tablets produced, draw and label a process flowchart, labeling masses of individual components rather than total masses and component mass fractions. It is unnecessary to label the stream between the mixer and the dryer. Carry out a degree-of-freedom analysis of the overall two-unit process.
(b) Calculate the masses and compositions of the streams that must enter the mixer to make 100,000 tablets.
(c) Why was it unnecessary to label the stream between the mixer and the dryer? Under what circumstances would it have been necessary?
(d) Go back to the flowchart of Part (a). Without using the mass of the wet powder (17.13 kg) or any of the results from Part (b) in your calculations, determine the mass fractions of the stream component~ in the powder fed to the mixer and verify that they match your solution to Part (b). (Hint: Take a basis of 100kg of wet powder.)
(e) Suppose a student does Part (d) before Part (b), and re-labels the powder feed to the mixer on the flowchart of Part (a) with an unknown total mass (m1) and the three now known mole fractions. (Sketch the resulting flowchart.) The student then does a degree-of-freedom analysis, counts four unknowns (the masses of the powder, pvp, and water fed to the mixer, and the mass of water evaporated in the dryer), and six equations (five material balances for five species and the percentage evaporation), for a net of -2 degrees of freedom. Since there are more equations than unknowns, it should not be possible to get a unique solution for the four unknowns.
2. Effluents from metal-finishing plants have the potential of discharging undesirable quantities of metals, Ch as cadmium, nickel, lead, manganese, and chromium, in forms that are detrimental to water and air quality. A local metal-finishing plant has identified a wastewater stream that contains 5.15 wt% chromium (Cr) and devised the following approach to lowering risk and recovering the valuable metal. The wastewater stream is fed to a treatment unit that removes 95% of the chromium in the feed and recycles it to the plant. The residual liquid stream leaving the treatment unit is sent to a waste lagoon. The treatment unit has a maximum capacity of 4500 kg wastewater/h. If wastewater leaves the finishing Plant at a rate higher than the capacity of the treatment unit, the excess (anything above 4500 kg/h) bypass the unit and combines with the residual liquid leaving the unit, and the combined stream goes to the waste lagoon.
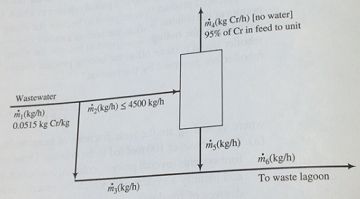
a) Without assuming a basis of calculation, draw and label a flowchart of the process.
b) Wastewater leaves the finishing plant at a rate m1 = 6000 kg/h. Calculate the flow rate of liquid to the waste lagoon, m6(kg/h), and the mass fraction of Cr in this liquid, x6(kg Cr/kg).
|
Interpret these banks credit quality over 2006-2014
: What is the difference between lending to individual borrowers via a residential home mortgage compared to other types of consumer lending
|
|
Database management and applications
: What errors prevent the table displayed above from being first normal form compliant? Bring the table(s) into first normal form compliance without loss of any data. Identify primary and foreign keys (when present) for all tables.
|
|
What would the mean and the variance equal
: Measured the tail lengths of 250 cats, and found that the average length is 28 cm and the variance is 75 cm2. If these tail lengths are normally distributed, (A) what would the mean and the variance equal if we multiplied each tail length by three? (..
|
|
How will the doppler shift in the radio signals detected
: How will the Doppler shift in the radio signals detected at planets A and B compare - how will the Doppler shift in the radio signals detected at planets E and B compare?
|
|
Draw and label a flowchart of the process
: Effluents from metal-finishing plants have the potential of discharging undesirable quantities of metals, Ch as cadmium, nickel, lead, manganese, and chromium, in forms that are detrimental to water and air quality. Without assuming a basis of ca..
|
|
Write a proposal to address problems faced by computers r us
: Write a research proposal to address the problems faced by Computers R Us. You are required to follow the following structure of the proposal?
|
|
A white ball was selected
: Consider 2 mugs. THe first contains 2 white and 7 black balls, and the second contains 5 white and 6 black balls. We flip a fair coin and then draw a ball from the first mug or the second mug depending on whether the outcome was heads or tails. What ..
|
|
If a and b are independent event
: If A and B are independent events, show the following (a) A and Bc are independent (b) Ac and Bc are independent
|
|
Credit card expenses
: You started recording every credit card transaction so that you can analyze your monthly expenses. You used an Excel worksheet to track dates, places, categories, and amounts. Because you are a consultant who travels periodically, you also have bu..
|