Reference no: EM131476014
Q1. Draw the Lewis structures for CO2 and CO, and predict the number of σ and π bonds for each molecule.
(a) CO2
(b) CO
Q2. What is the hybridization of the central atom in each of the following?
(a) BeH2
(b) SF6
(c) PO43-
(d) PCl5
Q3. Two important industrial chemicals, ethene, C2H4, and propene, C3H6, are produced by the steam (or thermal) cracking process:
2C3H8(g) ? C2H4(g) + C3H6(g) + CH4(g) + H2(g)
For each of the four carbon compounds, do the following:
(a) Draw a Lewis structure.
(b) Predict the geometry about the carbon atom.
(c) Determine the hybridization of each type of carbon atom.
Q4. For the carbonate ion, CO32-, draw all of the resonance structures. Identify which orbitals overlap to create each bond.
Q5. For each of the following structures, determine the hybridization requested and whether the electrons will be delocalized:
(a) Hybridization of each carbon
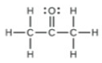
(b) Hybridization of sulfur
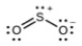
(c) All atoms
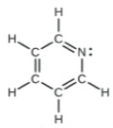
Q6. Calculate the bond order for an ion with this configuration:
(σ2s)2(σ*2s)2(σ2px)2(π2py,π2pz)4(π*2py,π*2pz)3
Q7. Predict the valence electron molecular orbital configurations for the following, and state whether they will be stable or unstable ions.
(a) Na22+
(b) Mg22+
(c) Al22+
(d) Si22+
(e) P22+
(f) S22+
(g) F22+
(h) Ar22+
Q8. For the first ionization energy for an N2 molecule, what molecular orbital is the electron removed from?
|
Define the principles of graphic design
: You have been asked to provide an expert review of the Kakadu National Park (Jabiru, Northern Territory, Australia) web site.
|
|
Prepare tables that show how firms estimated value changes
: You are a financial analyst at a leading company. Prepare tables that show how firm's estimated value changes as you vary the key inputs over relevant ranges.
|
|
What population does your hypothetical program serve
: What is the clinical area of concern (depression, anxiety, PTSD, eating disorders, self-esteem, et cetera) that your program is designed to address?
|
|
Comment on this insight by your friend
: When it is squared, the result is smaller, not bigger. Thus, having t2 instead of t in the formula makes the size of the tax less important.
|
|
Draw a lewis structure
: Two important industrial chemicals, ethene, C2H4, and propene, C3H6, are produced by the steam (or thermal) cracking process: Draw a Lewis structure
|
|
Why real wages are pro-cyclical
: The New Keynesians have their own 'story' as to why real wages are pro-cyclical. In the space below, write an essay explaining how the New Keynesians.
|
|
Why the two-party system has endured in the united states
: Which of the following is not a reason why the two-party system has endured in the United States? The Environmental Protection Agency is an example of a.
|
|
Analysis of the strengths and limitations of humanistic
: Explain how humanistic and existential theories influence interpersonal relationships. It is important to reflect behavior in your response.
|
|
Write a matlab script that plots the given functions
: Produce a single plot that displays the graphs of the functions sin(kx) across [0,211'], k = 1:5.
|