Reference no: EM131890358
Organic Chemistry Laboratory Experiments
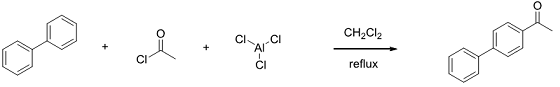
Experiment 1 - Friedel-Crafts Acetylation of Biphenyl
Please read Mohrig, pp 90-91: refluxing under anhydrous conditions; and pp 255-269: Thin Layer Chromatography. Watch the video on Blackboard about weighing moisture-sensitive solids. Complete the table below in your notebooks and as a pre-lab.
|
|
Biphenyl C12H10
|
Acetyl Chloride C2H3ClO
|
AlCl3
|
Methylene chloride CH2Cl2
|
C4H12O
|
|
MW (g/mol)
|
154.2
|
78.5
|
133.3
|
--
|
196.2
|
|
Qty. measured
|
mg
|
0.15 mL
|
Mg
|
6 mL
|
mg (theory)
|
|
Amount (mmol)
|
1.5
|
|
3.75
|
--
|
(theory)
|
|
Stoich. Equivs.
|
|
|
|
--
|
--
|
|
m.p. (oC)
|
|
--
|
--
|
(b.p.)
|
|
|
Dens. (g/mL)
|
--
|
|
--
|
--
|
--
|
The glassware needs to be dry. Please find the 15 mL round-bottom flasks and condensers in the oven and return them clean to the oven at the end of the experiment. As always, all glassware needs to be washed first with water, then acetone.
Collect the crude solid into a pre-tared vial and determine the crude yield. Label the vial with name, section, and contents. You will need this material for the following week.
Before leaving the lab, hand in to your instructor the labeled vial with your crude product to be purified the following week. Have your instructor check the quantity of crude product and your notebook including:
1. Diagram of TLC plate.
2. Calculations of Retention Factor (Rf) values of all TLC spots.
3. Calculation of crude yield.
Experiment 2 - Purification of 4-Acetyl-Biphenyl Using Column Chromatography
Objective: To purify 4-acetyl-biphenyl from the other bi-products of the Friedel-Crafts reaction. Flash column chromatography will be used, in conjunction with thin layer chromatography on the collected fractions to assess the extent of separation of the components of the crude mixture.
QUESTIONS to be answered on Formal Lab report:
1) Draw a detailed mechanism (with arrow-pushing) for the Friedel-Crafts acetylating of biphenyl; include the resonance forms of the reaction intermediate produced.
2) Draw the product of di-acetylation of biphenyl. Explain why acetylation does not occur twice on the same ring.
Attachment:- Assignment Files.rar