Reference no: EM13840111
A. In Lewis theory, the two bonds in a double bond look identical. However, valence bond theory shows that they are not.
1. Describe a double bond according to valence bond theory.
2. Explain why rotation is restricted about a double bond, but not about a single bond.
B. The following figures show several molecular geometries (Express your answers as integers separated by commas.)
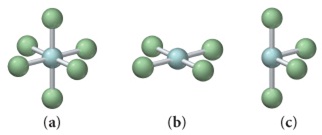
1. Give the number of total electron groups, the number of bonding groups, and the number of lone pairs for (a) geometry.
2. Give the number of total electron groups, the number of bonding groups, and the number of lone pairs for (b) geometry.
3. Give the number of total electron groups, the number of bonding groups, and the number of lone pairs for (c) geometry.
B. Determine the electron geometry, molecular geometry, and idealized bond angles for each of the following molecules. In which cases do you expect deviations from the idealized bond angle?
(a) CF4
(b) NF3
(c) OF2
(d) H2S
1. Determine the electron geometry for each molecule: linear, tetrahedral, trigonal planar, trigonal bipyramidal
2. Determine the molecular geometry for each molecule: tetrahedral, trigonal planar, bent, linear
3. Determine the idealized bond angle for each molecule: 90. 180. 109.5, 120
4. In which cases do you expect deviations from the idealized bond angle?1. Determine the molecular geometry of BrF5.
a. seesaw
b. trigonal bypyramidal
c. square pyramidal
d. octahedral
2. Make a sketch of BrF5, using the bond conventions shown in the Box in Section 10.4 in the textbook. Draw the molecule, with the correct chirality, by placing atoms on the grid and connecting them with bonds.
3. Determine the molecular geometry of SCl6.
- seesaw
- trigonal bipyramidal
- square pyramidal
- octahedral
4. Make a sketch of SCl6.Draw the molecule, with the correct chirality, by placing atoms on the grid and connecting them with bonds.
5. Determine the molecular geometry of PF5.
- seesaw
- trigonal bipyramidal
- square pyramidal
- octahedral
6. Make a sketch of PF5.
Draw the molecule, with the correct chirality, by placing atoms on the grid and connecting them with bonds.
7. Determine the molecular geometry of IF+4.
- seesaw
- trigonal bipyramidal
- square pyramidal
- octahedral
8. Make a sketch of IF+4.
Draw the molecule, with the correct chirality, by placing atoms on the grid and connecting them with bonds. Show the formal charges of all atoms in the correct structure.
9.
D. Each of the following ball-and-stick models shows wrong electron and molecular geometry of a generic molecule.
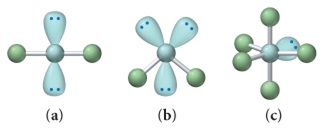
1. Provide the correct molecular geometry for (a), given the number of lone pairs and bonding groups on the central atom.
- octahedral
- tetrahedral
- trigonal planar
- trigonal pyramidal
- square pyramidal
- seesaw
- bent
- linear
- trigonal bipyramidal
2. Provide the correct molecular geometry for (b), given the number of lone pairs and bonding groups on the central atom.
- trigonal planar
- trigonal bipyramidal
- octahedral
- square pyramidal
- bent
- seesaw
- trigonal pyramidal
- linear
- Tetrahedral
3. Provide the correct molecular geometry for (c), given the number of lone pairs and bonding groups on the central atom.
- seesaw
- octahedral
- bent
- linear
- tetrahedral
- trigonal planar
- square pyramidal
- trigonal pyramidal
- trigonal bipyramidal
E. ..
1. How do you determine whether a molecule is polar?
2. Why is polarity important?
F. CH3F is a polar molecule, even though the tetrahedral geometry often leads to nonpolar molecules.
1. Draw the Lewis structure of CH3F.Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and all hydrogen atoms.
G. Determine whether each molecule given below is polar or nonpolar.
1. CH2Cl2
2. PBr5
3. XeF2
4. H2S
H. Determine whether each of the following molecules is polar or nonpolar.
1. SiCl4
2. CO2
3. SeF6
4. XeF2
I. What is a chemical bond according to valence bond theory?
J. The valence electron configurations of several atoms are shown. How many bonds can each atom make without hybridization?
1. Mg3s2
2. N2s22p3
3. O2s22p4
K. Consider the structure of the amino acid aspartic acid. Indicate the hybridization about each interior atom: sp sp^2, sp^3
L. Use the drawing of MO energy diagram to predict the bond order of Li2+ and Li2-.
1. Do you expect Li2+ to exist in the gas phase?
yes
no
2. Do you expect Li2- to exist in the gas phase?
yes
no
M. Use molecular orbital theory to predict whether or not each of the following molecules or ions should exist in a relatively stable form.
1. C2+2
will exist
will not exist
2. Li2
will exist
will not exist
3. Be2+2
will exist
will not exist
4. Li2-2
will exist
will not exist
N. Use the drawing of energy diagram for HCl to predict the bond order.
Express your answer using two significant figures.
O. Draw Lewis structures for CN+, CN, and CN-.
1. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons. Show the formal charges of all nonhydrogen atoms in the correct structure.
2. Draw Lewis structure for CN.Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons.
3. Draw Lewis structure for CN-.Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons. Show the formal charges of all nonhydrogen atoms in the correct structure.
4. According to Lewis theory, which species is most stable?
CN+
CN
CN-
5. According to MO theory, which species is most stable?
CN+
CN
CN-
P. Classify each of the following compounds as organic or inorganic.
1. C8H18
organic
inorganic
2. H2CO3
inorganic
organic
3. CaO
inorganic
organic
4. C12H16N2O
organic
inorganic
Q. Classify each of the following hydrocarbons as an alkane, alkene, or alkyne.
1. HC≡CH
alkane
alkene
alkyne
2. H3C-CH=CH-CH3
alkane
alkene
alkyne
3. CH3-C-CH3H-CH3
alkane alkene alkyne
4. H3C-C≡C-CH3
alkane
alkene
alkyne