Reference no: EM131146215
The is an Analytical Methods Exam.
The answers to these questions are to be short and concise.
Please only answer do not add any additional information other than what the question is asking. Please keep any diagrams or mechanisms as as simple as possible.
Analytical Methods
Section A
(a) Define and contrast the following terms used in chromatography.
(i) Retention time (tR)
(ii) Capacity factor (k')
(d) Discuss the effects of column length on analysis time and resolution in isothermal and temperature-programmed GC analysis.
Section B
(a) Assign the protons in the molecules 1 and 2 below to their appropriate 1H NMR spin systems using the simplified Pople convention.1 2
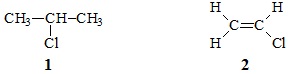
(b) Predict and sketch the precise appearance of the 1H NMR spectrum (assume 1st order appearance) of compound 2 in part (a) above. Your answer should include an illustrative splitting tree diagram to establish the appearance of one of the signals, as well as all anticipated chemical shifts derived from NMR parameter tables.
(c) A sample taken from the scene of a suspected suicide is believed to contain aspirin (structure below) and a sample of it is dissolved in D2O and subjected to 1H NMR analysis.
Using NMR parameter tables (Provided at the bottom for this Exam Paper) to help estimate chemical shifts wherever possible, deduce and describe with the aid of sketches the spectrum that would be expected if the sample was indeed aspirin.
State clearly the intensity and chemical shift of each signal and describe clearly any splitting patterns or other diagnostic features of the spectrum.
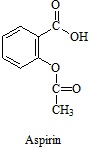
You may ignore any long-range splitting effects but you should comment on the likelihood of 2nd order appearance in the predicted spectrum.
Aspirin
Section C
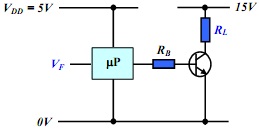
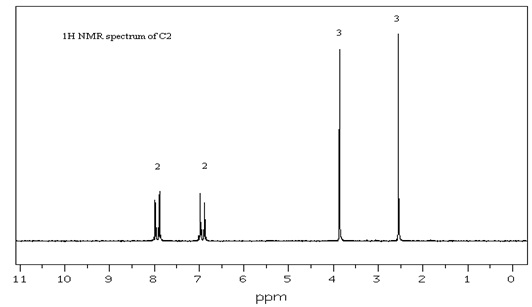
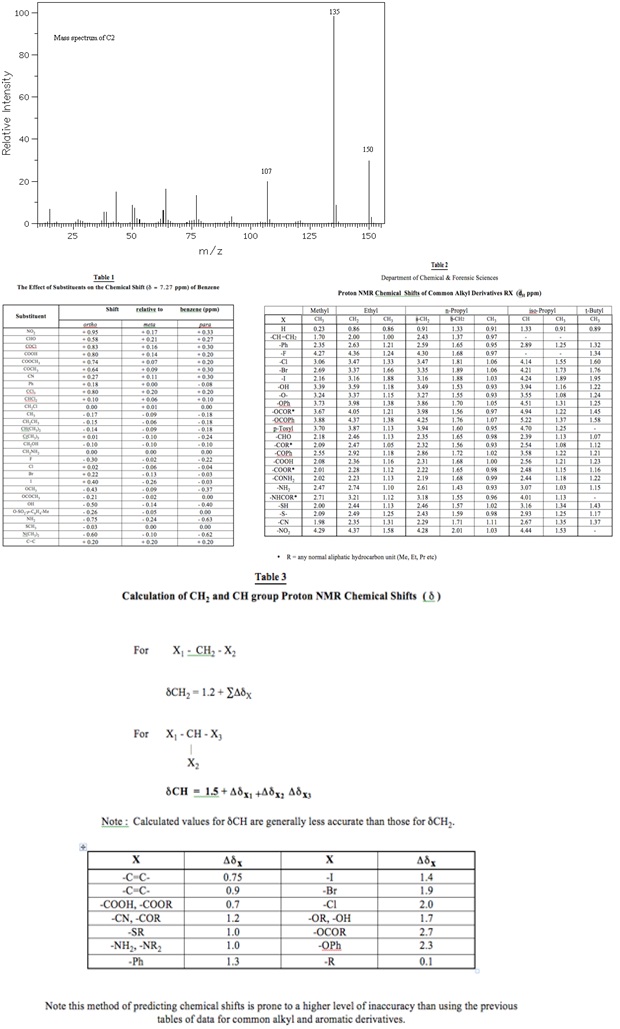
A. Deduce the molecular structure of compound C2 (C9H10O2) from the spectra provided below and overleaf. Explain clearly your reasoning at each stage in the deduction process and explain each diagnostically significant signal in each spectrum.