Reference no: EM13886230
Discuss the bonding in XeF2 from BOTH the VB theory and the MO theory point of view.
Speci?cally:
For valence bond theory (VBT):
Draw the best Lewis structure
Give the ground state electronic con?guration of xenon (central atom)
Give the valence state electronic con?guration of xenon (what changes in order for bonding to occur?) Which atomic orbitals (hybrid orbitals) on Xe are used for bonding?
Give the hybridization on Xe in XeF2 (central atom).
For molecular orbital theory (MOT):
Determine the point group of the molecule.
Determine the symmetry of the atomic orbitals on the central atom (Xe)
Determine the symmetry of the ligand group orbitals (LGO) (2 F atoms).
Draw a qualitative MO diagram with the Xe AOs on the left and the LGOs on the right side.
Which orbitals have the correct symmetry (and energy) to form bonding (and anti-bonding) MOs? The LGOs formed from 2s orbitals are low in energy, i.e. they do not form bonds with the central atom. Be sure to include all AOs and LGOs even if they are non-bonding.
When LGOs do not form bonds, what are those orbitals named? (What are those orbitals labelled?) (Clearly label all AOs, LGOs and MOs in your MO diagram)
Connect lines between appropriate orbitals.
Fill the MO diagram with the appropriate number of electrons.
Discuss the two results. What is the bond order in each? Does VBT and MOT agree? Disagree? Give the HOMO and LUMO.
Consider the following blank (next page) molecular orbital diagram for NO2. Completely label each of the atomic and ligand group orbitals as well as the molecular orbitals with the appropriate symmetry labels. Label the bonding MO's as sigma or bay
Example to start: (s + s) is totally symmetric (symmetry must be a1)
Use the orbital information above to help:
Ligand group orbitals for the oxygen atoms in NO2, derived from the 2s and 2p atomic orbitals. The z axis is de?ned along the N-O bond for each.
The x axis is still perpendicular to the plane of the molecule and the y axis must be mutually perpendicular to both x and z axes.
Consult the appropriate character table and determine the symmetry for each of the LGO's (above). Use this information to complete the MO diagram on the next page. Clearly label all AOs, LGOs and MOs with the correct symmetry (Mulliken symbol). Clearly connect appropriate orbitals.
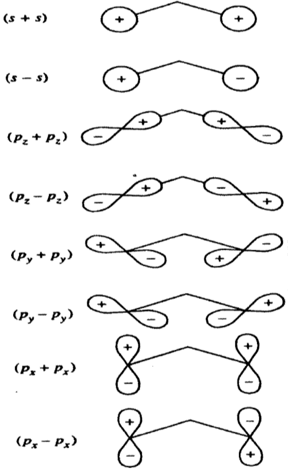
(If you prefer to create entire MO diagram, that is also acceptable (rather than using the outline above))
1: Fill the levels with the appropriate number of electrons. Label the HOMO and LUMO.
(a) Give the molecular orbital electron configuration for NO2.
(b) What is the bond order?
(c) Is NO2 (not nitrite) paramagnetic or diamagnetic?
(d) Compare the stability of NO2, NO2-, NO2-, NO2+
Consider the molecular orbital diagram on the next page:
Deubel, JACS, 2002, 124, 12312-12318 Krossing's Cation
The left side of the ?gure includes only the s and d orbitals on the central atom (silver); the right side of the ?gure shows the interactions for the p orbitals.
According to the published paper, the symmetry of the silver P4 complex is D2h or D2d (see Figure 1 in paper); the silver ole?n complex is studied for comparison (similar geometries: D2h or D2d)(see Figure 5 in paper). Using what you have learned about SALC and MO diagrams, discuss the molecular orbital diagram. For example, the symmetry designations for the central atom (silver) are not given, but have been used (con?rm the correct symmetry labels).
(There is at least one error in the ?gure (in Dr. A-K's opinion); discuss any errors you ?nd.)
Do you agree with the conclusions of the author? Why or why not? (Do NOT include a discussion of DFT. Focus on the bonding, SALC, MO
concepts discussed in class.)