Reference no: EM131412061
Assignment
You will need to refer to character tables to answer some questions. Character tables are in appendix C, pp 658-666, of the text. If you need additional space, use the back side of a page or attach additional sheets.
1. Consider the following tetrahedral metal complexes:
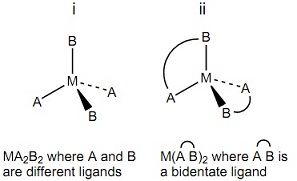
a. Determine the point group of each complex.
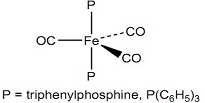
M(A B)2 where A B is a bidentate ligand
b. Classify each complex as chiral or achiral based on its point group. If a complex is chiral, indicate whether it is asymmetric or dissymmetric.
2. Sulfur trioxide, SO3, is a trigonal planar molecule with D3h symmetry
a. Determine the number of normal (fundamental) vibration modes for this molecule.
b. Normal coordinate analysis gives the following symmetries for the vibrational modes: A1', A2", E', E'. Is this total consistent with the result in ‘a'? Explain. (Each E symmetry is doubly degenerate meaning two vibration modes with the same energy.)
c. Which of these symmetries are IR active?
d. How many fundamental absorptions should be observed in the IR spectrum of SO3?
e. The nitrate ion, NO3-, is trigonal planar. How many fundamental absorptions should be observed in the IR spectrum of NO3- ? Explain.
3. [Ni(CO)4] is a tetrahedral molecule. Shown below is the reducible representation for CO stretches in the Td point group. The characters correspond to the number of COs that remain in place (are not shifted) by application of the indicated symmetry operation
|
Td
|
E
|
8C3*
|
3C2*
|
6S4*
|
σd
|
|
Γ
|
4
|
1
|
0
|
0
|
2
|
*As discussed in class, the number preceding the operation corresponds to sequential (e.g. C3 and C32) or directional (clockwise and counter clockwise), or multiple operations (e.g. four C3 axes in the Td point group) which belong to the same symmetry class and hence, have the same characters.
a. Which two irreducible representations (symmetries) in the Td point group add to give the reducible representation? Confirm this, by showing that the sum of characters of each symmetry operation in the component irreducible representations add to give the corresponding character in the reducible representation.
b. How many IR active CO stretching modesare there in the Td point group? Indicate by symmetry label.
c. How many Raman active CO stretching modes are there in the Td point group? Indicate by symmetry label.
4a. Determine the symmetries of the CO stretching modes in trans-Fe(CO)3(P(C6H5)3)2. This molecule belongs to the D3h point group. First construct the reducible representation by determining the number of COs that remain in place (are not shifted) by the application of the each symmetry operation as shown for the S3 operation:
|
D3h
|
E
|
2C3*
|
3C2*
|
σh
|
2S3*
|
3σv
|
|
G
|
|
|
|
|
0
|
|
b. Next, determine, by inspection, the irreducible representations (symmetries) that this reducible representation reduces to. Confirm this, by showing that the sum of the characters of each symmetry operation in the component irreducible representations add to give the corresponding character in the reducible representation.
c. How many IR active CO stretching modes are there in trans-Fe(CO)3(P(C6H5)3)2? Indicate by symmetry label.
5. Determine the number of IR active CO stretching modes for the following isomer of Fe(CO)4(P(C6H5)3):

This is just like problems 3 and 4 above except the point group is not given. Start by: i) determining the point group; ii) then construct the reducible representation by determining the number of COs that remain in place by application of each symmetry operation in the point group; remember that the 'z' axis is coincident with the highest fold rotational axis; iii) next, determine, by inspection, the irreducible representations (symmetries) that this reducible representation reduces to. Confirm this, by showing that the sum of the characters of each symmetry operation in the component irreducible representations add to give the corresponding character in the reducible representation; iv) finally, identify the IR active CO stretches by symmetry label.
This analysis needs to be carried out in the given sequence. Additionally, the point group assignment (step i) determines which symmetry operations are utilized in constructing the reducible representation and so forth. Thus, an incorrect point group assignment (step i) will translate into an incorrect reducible representation (step ii) which will translate into an incorrect set of irreducible representations (step iii) and most likely, an incorrect number of CO stretches (step iv).