Reference no: EM131394066
Instructions: Solve the following problems, showing all the steps you made in the procedure.
Problem 1:
A column packed with 50 mm in ceramic Pall ring is to be design for the following vapor and liquid conditions:
Data:
|
|
Vapor
|
Liquid
|
|
Mass flow rate , kg/h
|
525
|
1360
|
|
Density, kg/m3
|
1.182
|
1000
|
|
Viscosity, kg/m-s
|
1.78x10-5
|
1.00x10-3
|
|
Molecular weight
|
28.4
|
18.02
|
|
Surface tension, kg/s2
|
|
2.401x10-2
|
The column is operated at 110 kPa and 303 K. The process carried out with 69 percent of the flooding velocity.
Units of kinematic viscosity are m2/s.
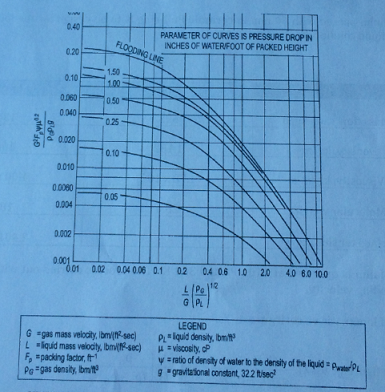
Use the figure (Y vs. X): G2FPμL0.1ψ/ρG(ρL-ρG)gc vs. L'/G'(ρG/ρL-ρG)0.5
Determine:
1. Molar flow rate.
2. The vapor and liquid superficial velocities at the loading and flooding points.
3. The void fraction.
4. The specific liquid holdup at the loading point.
5. The specific pressure drop at the loading and flooding point.
6. The column inside diameter for operation at the loading point.
7. The dry-gas pressure drop (Pa/m).
8. The liquid and gas mass velocity (kg/m2-s).
9. kL and ky.
10. The volumetric gas-phase and liquid phase mass transfer coefficients.
11. HTU (HtG)
12. NtG
13. Packed height, Z in m.
14. Give some characteristics from the packing used in this design.
Problem 2: Design of a sieve tray column
Carbon disulfide, CS2, used as a solvent in a chemical plant, is evaporated from the product in a dryer into an inert gas (essentially N2) in order to avoid an explosion hazard. The CS2-N2 mixture is to be scrubbed with an absorbent hydrocarbon oil (C18H38). The gas will flow at the rate of 0.45 m3/s at 297 K and 1 atm. The partial pressure of CS2 in the original gas is 55 mmHg, and the CS2, concentration in the outlet gas is not to exceed 0.55%. The oil enters the absorber essentially pure at a rate 1.3 times the minimum, and solutions of oil and CS2 follow Raoult.s law.
Give a sketch of the countercurrent tower.
|
|
MM
|
ρ (kg/m3)
|
P* (mmHg)
|
μ
|
σL (N/m)
|
Difussivity (cm2/s)
|
|
Oil
|
254
|
810
|
|
4 cP
|
0.030
|
0.765x10-5
|
|
Gas
|
|
|
346
|
1.7x10-5 (kg/m-s)
|
|
0.114
|
Foaming factor: 0.85
Tray spacing (t): 0.5 m
Hole diameter (d0): 5 mm
Equilateral-triangular pitch: 12 mm centers punched in sheet metal.
Stainless steel metal: 2.5 mm thick
Design for a gas velocity which is 75% of the flooding velocity.
hw: 5.0 cm
LSmin is 5.95 mol/s
Assume isothermal operation, 297 K.
Assume equilibrium and operating lines. Graph Y vs X.
Results: Give all results in a
Liquid flow rate (kg/s):
Diameter (m):
Plate spacing (m):
Total cross-sectional area (m2):
Downcomer area (m2):
Details of the tray design:
Active area over the tray (m2):
Weir length (m):
Distance from tray center to weir (m):
Total hole area (m2):
hd (cm):
hI (cm):
hσ (cm):
ht (cm):
ΔPG (Pa/tray)
Fr0:
Entrainment (E):
Entrainment mass flow rate (kg/s):
EOG:
EMG:
EMGE:
Number of ideal trays.
Number of real trays required.
Give graphical solution and analytical solution
Conversions:
1.0lbm = 453.6 g
1.0 m= 3.2808 ft
1.0 m3 = 35.31 ft3
g = gc (in American system)
1 cP = 0.001 Pa-s
1.0 dyne = 0.00001 N
|
What is the bond''s current value
: Eight years ago, Over-the -Top Trampolines issued a 15-year bond with a $1,000 par value and a 6 percent coupon rate (interest is paid annually). Today the going rate of interest on similar bonds is 6 percent.
|
|
Compare the observed behaviors with those of human beings
: Compare and contrast the observed behaviors with those of human beings. Are there similar behavior patterns in humans? Why or why not?
|
|
Explain foreign exchange market
: Explain foreign exchange market. Write about all the types of foreign exchange Markets. Explain the participants in foreign exchange markets.
|
|
Expulsion or suspension of members from professional
: Why is it imperative for you to become familiar with the guiding principles of the ethics codes and accepted standards of practice of your profession?According to the text, which of the following would not be considered a professional mental health..
|
|
Determine the molar flow rate
: A column packed with 50 mm in ceramic Pall ring is to be design for the following vapor and liquid conditions: Determine: 1. Molar flow rate. The vapor and liquid superficial velocities at the loading and flooding points. The void fraction.
|
|
Sexual or romantic feelings toward a client
: Shania is seeking counseling to resolve her feelings about the ending of an extramarital affair. Her boyfriend who is married broke up with her and she is very angry about this. She informs her therapist that she plans to contact the wife in order..
|
|
Discuss the goals of international financial management
: Discuss the goals of international financial management. The key component of the financial system is the money market that acts as a fulcrum of monetary operations.
|
|
Working effectively with cultural diversity
: The APA has developed a set of specialty guidelines for psychotherapy with lesbian, gay, and bisexual clients that prohibit unfair discrimination based on sexual orientation. Which of the areas stated below are not mentioned in the guidelines?
|
|
What is the bond’s yield to maturity
: Gabby's Garage issued a bond with a 10-year maturity, a $1,000 par value, a 10 percent coupon rate, and semiannual interest payments.
|