Reference no: EM13800687
Problem 1. A closed rigid tank whose volume is 1.5 m3 contains Refrigerant 134a, initially a two phase liquid vapor mixture at 10 °C. The refrigerant is heated to a final state where temperature is 50°C and quality is 100%. Locate the initial and final states on a sketch of the ?? - ?? diagram. Determine the mass of vapor present at the initial and final states, each in kg.
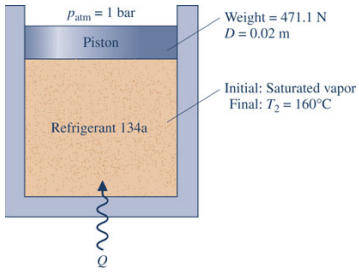
Problem 2. Refrigerant 134a is contained in a piston-cylinder assembly, initially as saturated vapor the refrigerant is slowly heated until its temperature is 160°C. During the process, the piston moves smoothly in the cylinder. For the refrigerant, evaluate the work per unit mass, in kJ/kg.
Problem 3. A horizontal piston-cylinder assembly (closed system) contains 0.1 kg of water, initially at 1 MPa, 500 °C. The water undergoes two processes in series: Process 1-2: Constant-pressure cooling by compression until the volume becomes half of the initial volume. And point 2 is a mixture of vapor and liquid. Process 2-3: Constant-volume cooling by heat transfer until the water cools to 25°C.
(1) Sketch process 1-3 on a T-υ diagram.
(2) Neglect change of kinetic and potential energy, find the work (??1-2) and heat transfer (??1-2) in kJ for process 1-2.
(3) Neglect change of kinetic and potential energy, find the work (??2-3) and heat transfer (??2-3) in kJ for process 2-3.
Problem 4. A closed, rigid tank is filled with a gas modeled as an ideal gas, initially at 27 °C and a gage pressure of 300 kPa. If the gas is heated to 77°C, determine the final pressure, expressed as a gage pressure in kPa. The local atmospheric pressure is 1 atm.
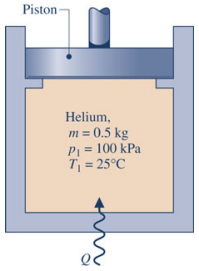
Problem 5. A piston-cylinder assembly whose piston is resting on a set of stops contains 0.5 kg of helium gas, initially at 100 kPa and 25 °C. The mass of the piston and the effect of the atmospheric pressure acting on the piston are such that a gas pressure of 500 kPa is required to raise it. How much energy must be transferred by heat to the helium, in kJ, before the piston starts rising? For the helium, assume ideal gas behavior with a constant ???? = 5 2 ??. Assume ???? is a constant at all temperatures.
|
Determining the likelihood
: Create a detailed Risk Assessment for the Project Team. Assessing each risk involves determining the likelihood that the risk eent will occur and the degree of impact the event will have on the project. Each of these factors can be assigned a rating..
|
|
Quality-improvement plan for the organization
: Create aMicrosoft® PowerPoint® or Prezi® presentation on the quality-improvement plan for the organization chosen by the Learning Team for the Influencing and Controlling the Project assignment.
|
|
Relationship between tasks in a project plan
: Why is it important to identify the relationship between tasks in a project plan? Provide an example of when you would create a project schedule with some slack and explain why.
|
|
What is the intrinsic value of the companys common stock
: Whats the intrinsic value of the stock based on the required rates of return - What is the intrinsic value of the companys common stock?
|
|
Determine the mass of vapor present at the initial states
: A closed rigid tank whose volume is 1.5 m3 contains Refrigerant 134a, initially a two phase liquid vapor mixture at 10 °C. The refrigerant is heated to a final state where temperature is 50°C and quality is 100%. Locate the initial and final state..
|
|
How institutional racism is present in many urban schools
: How institutional racism is present in many urban schools
|
|
Assignment on psychological egoism
: Psychological Egoism, After reviewing Chapter 1 in your textbook and watching "Virtue Ethics," find a contemporary article showing how the theory of psychological egoism in a corporation resulted in an ethical dilemma.
|
|
Implications of resource allocation
: What are some implications of resource allocation when an organization is involved in several projects at once? Why do you suppose that the coordination of the various elements of the project is considered the most difficult aspect of project exec..
|
|
Write essay about observations on the future of productivity
: write an organized and well-supported essay in which you make three observations on the future of productivity. Do you believe the future is optimistic?
|