Reference no: EM13368
Production of Chemicals from Coal
Coal has been considered a major component of U.S. energy reserves for a long time, and more recently it has become an important raw material for the production of chemicals.
The most common coal gasification process involves the reaction of carbon, the primary constituent of coal, with steam to produce carbon monoxide and hydrogen.
C + H2O → Co + H2 (1)
The carbon monoxide and hydrogen can be separated from the other compounds exiting the gasifier and used either as a fuel or a feedstock to a chemical manufacturing process. Simultaneously, oxygen fed to the reactor along with steam reacts with carbon to form carbon dioxide.
C+O2 → CO2 (2)
In addition, there is significant reaction between water and carbon monoxide in the gasifier via the water-gas shift reaction.
CO+H2O ↔ CO2 + H2 (3)
Although Reactions 1, 2 and 3 are the primary reactions in coal gasification, there are important secondary reactions. For example, the hydrogen-rich atmosphere in the gasification reactor causes the formation of hydrogen sulfide from the sulfur usually found in coal and of small amounts of methane.
S + H2 → H2S
C + 2H2 → CH4
Table: Distribution of Proven Coal Reserves In Billions of Tons
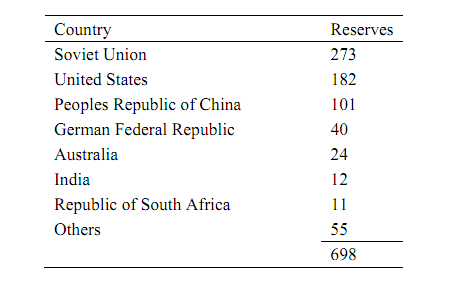
Your company has plans to build a plant to convert coal to acetic anhydride, a chemical vital to the company's product line. The plant is to produce 160,000 metric tons of acetic anhydride per year. It is anticipated that the completed process will operate continuously 350 days/ yr. The composition of coal from the nearest deposit is given in Table 2. Judging from the sulfur content, production of significant quantities of H2S is expected. Since H2S poisons (deactivates) catalysts used in the conversion of hydrogen and carbon monoxide into acetic anhydride, it must be removed from the gas leaving the gasifier. It is your responsibility to assist with the setting of design variables necessary for sizing equipment.