Reference no: EM131343677
Question 1. A control valve is to be used to control water flow at a maximum rate of 120 US gallons per minute. If the maximum pressure drop across the valve is 100 psi, determine the valve size required for this particular application. The table below relates CV and valve size.
|
CV
|
Valve size (inches)
|
|
0.3
|
1/4
|
|
3
|
1/2
|
|
14
|
1
|
|
35
|
1.1/2
|
|
55
|
2
|
|
108
|
3
|
|
174
|
4
|
|
400
|
6
|
|
725
|
8
|
TABLE 1
Question 2. FIGURE 1 shows the valve characteristics of three different types of valve trim.
FIG. 1
(i) Identify each of the three characteristics.
(ii) State, with reasons, a typical type of application for each of the three types of valve trim.
Question 3. Write brief notes on the following topics:
(a) flashing in control valves
(b) cavitation in control valves
(c) control valve noise
(d) reasons for the use of valve positioners.
Question 4. The level of liquid in an open tank (FIGURE 2) is to be controlled by regulating the flow of liquid into the vessel.
(a) Complete the drawing to show how the level can be controlled by linking the liquid inflow and liquid level controls in cascade.
(b) Draw a control block diagram of the cascaded system.
(c) Describe the response of the cascaded system to a change in the measured level of liquid.
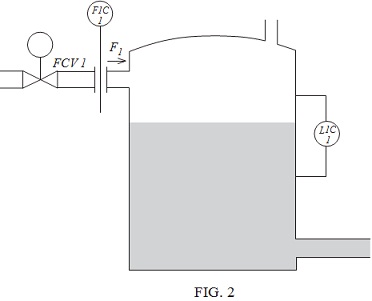
Question 5. FIGURE 3 shows the block diagram of the control of an electric heating system. The heater is driven from a voltage-controlled power supply, the voltage V1 being derived from a potientiometer. The output temperature, θO, is subject to disturbances, θD, because of changes in the ambient temperature. It is proposed to apply 'disturbance feedback control' to the system by the inclusion of a transducer that measures the external temperature and feeds a signal back to the input via a proportional controller of gain H.
Determine the required value of H to eliminate the effect of the disturbance.
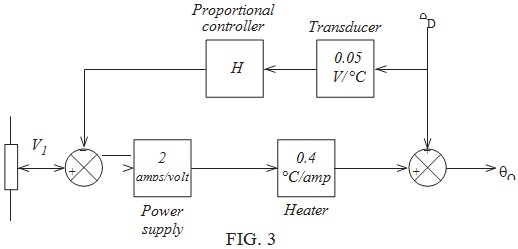
Question 6. The purpose of the arrangement shown in FIGURE 4 is to mix the two liquid products A and B in a fixed mass ratio. Product A, which is itself a mixture, is a 'wild' flow, whilst product B, a pure compound, is controlled. As the mixture leaves the tank the transmitter ρTX measures its density.
(a) Complete the diagram to show how the arrangement could be controlled by the method of 'variable ratio control'.
(b) Identify which transmitter provides 'feedforward'.
(c) Describe how the control system responds to a disturbance caused by a variation in the density of product A.
Question 7. FIGURE 5 shows a partially completed diagram of a flow control system. The flow controller is reverse acting and has a 0.2 to 1.0 bar pneumatic output signal which will supply both control valves V1 and V2.
The small range control valve, V2, only needs to operate on the first 25% output change of the controller output signal. For larger flow rates the small range valve will remain fully open and control will be achieved by operation of the large range valve. Note the differing air failure action of the two valves.
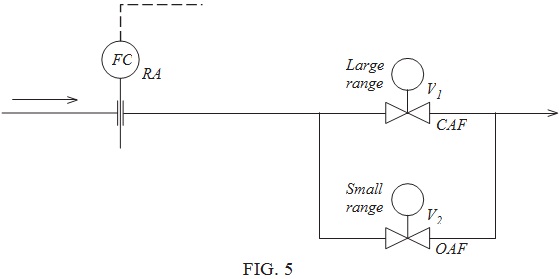
Design a system utilising valve positioners which will meet the prestated specifications.
|
What is the molarity of the koh solution
: 1. A buffer system contains 0.18 MNH4+ and 0.14 M NH3. pKa of NH4+ is 9.25. How many moles of NaOH must be added to 1.00 L of this solution to increase the pH to 9.25? 2. Titration of a 15.0 mL solution of KOH requires 17.0 mL of 0.0250 M H2SO4 ..
|
|
Share price-all equity plan and levered plan
: Franklin Corporation is comparing two different capital structures, an all-equity plan (Plan I) and a levered plan (Plan II). Under Plan I, the company would have 185,000 shares of stock outstanding. What is the value of the firm under each of the tw..
|
|
Final temperature after thermal equilibrium
: A 200 g sample of water with a temperature of 32?C is added to 50 g water at 55?Cin an insulated container. What is the final temperature after thermal equilibrium is reached?
|
|
Analyze the rationale for health insurance expansion
: Analyze the rationale for health insurance expansion. Include how health insurance expansion relates to problem of adverse selection. What are the economic implication of health insurance expansions for health care organizations?
|
|
Design a system utilising valve positioners
: Design a system utilising valve positioners which will meet the prestated specifications - Complete the diagram to show how the arrangement could be controlled by the method of 'variable ratio control' and Identify which transmitter provides 'feedfor..
|
|
Discuss about the passive and active thermography
: Describe in your own words (not more than 250 words) the differences between passive and active thermography. List at least 2 typical applications of each technique . A double pane window has an inside temperature of 20C and an outside temperature..
|
|
What is the adjusted present value of project
: Zoso is a rental car company that is trying to determine whether to add 25 cars to its fleet. The company fully depreciates all its rental cars over six years using the straight-line method. What is the maximum price that the company should be willin..
|
|
Are the allocation bases suggested in the case reasonable
: Case - Harbor City Electric. Are the allocation bases suggested in the case "reasonable"? If not, how would you change them? Which of the two bases under discussion for the maintenance and repair cost center would you use? Why
|
|
Why do you feel that is an important question
: Imagine you can interview the presenters and ask one question about financial risks and rewards. What question would you ask? Why do you feel that is an important question?
|