Reference no: EM131034278
Reactor Engineering-
Q1. Describe the physical motion of the fluid and the main modes of heat transfer in each section (I through IV) of the following figure.
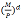
Q2. Write the radial thermal resistance equation between Tf and Tm for the following annular geometry? In the solution equation, there should not be T1, T2, T3 and T4
Where
di: the diameter of ith element
Tf: coolant temperature
Tm: coolant temperature
Ti: the surface temperature of each annular layer
Q: Transferred heat
ki: conductivity coefficient of element i
ha and hb: convective heat transfer coefficients for inner and outer surfaces
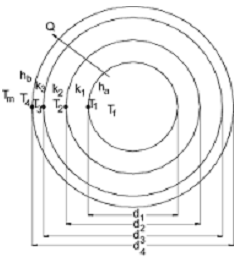
Q3. By using the following control diagram, explain why feedback effects are important? In addition, explain each component in this figure and how these components are connected to each other.
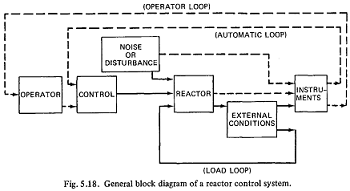
Q4. The figure on left hand side shows the axial power profile of a fuel pin. The figure on right hand side shows the temperatures of fuel surface and coolant. Based on this figures, explain why not maximum surface temperature happens at L/2 and the peak point of "ts" shifts to right?
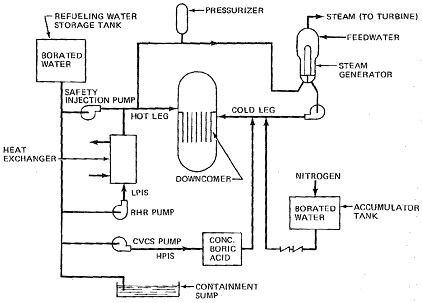
Q5. The following figures shows the ECCS of a traditional PWR. Please explain how ECCS of PWR works?
|
Sulfur to remove a valence electron
: Why would you expect phosphorous to require less ionization energy than sulfur to remove a valence electron?
|
|
How it specifically impacts the creation of digital media
: Describe each practice, explain why it is a best practice, and then relate it to how it specifically impacts the creation of digital media within chosen specialization, as opposed to only providing general content that could apply to any digital m..
|
|
Although aluminum chloride alcl3 is ionic
: Although aluminum chloride AlCl3 is ionic in the solid state is molecules in the gaseous State write the Lewis structure prepare a VSEPR sketch describe the geometry prepare a VB sketch and describe the bonding in gaseous AlCl3
|
|
Determine the heat transfer during this process
: A piston-cylinder device contains 6 kg of H2 and 21 kg of N2 at 160 K and 5 MPa. Heat is now transferred to the device, and the mixture expands at constant pressure until the temperature rises to 200 K.
|
|
Describe the physical motion of the fluid
: Describe the physical motion of the fluid and the main modes of heat transfer in each section (I through IV) of the following figure
|
|
Determine the average molar mass of the mixture
: Determine the average molar mass of the mixture, the average specific heat at constant pressure of the mixture at 600 K, in kJ/kmol -K, and the partial pressure of the water vapor in the mixture for a mixture pressure of 200 kPa.
|
|
A power of attorney is best described
: A "power of attorney" is BEST described as:A. An oral employment contract between a principal an agent
|
|
Some agents are classified as servants
: Some agents are classified as "servants" while others are classified as "independent contractors." Which of the following best describes the main difference between these two kinds of agents?
|
|
Develop a valid musical aptitude test than a reliable one
: Describe how you would standardize your test and assess reliability and validity. Explain why it may be more difficult to develop a valid musical aptitude test than a reliable one.
|