Reference no: EM131506008
Assignment
1.Write the electron configurations for each of the following elements and its ions:
(a) Ti
(b) Ti2+
(c) Ti3+
(d) Ti4+
2. The following reactions all occur in a blast furnace. Which of these are redox reactions?
(a) 3Fe2 O3(s) + CO(g) → 2Fe3 O4(s) + CO2(g)
b) Fe3 O4(s) + CO(g) → 3FeO(s) + CO2(g)
c) FeO(s) + CO(g) → Fe(l) + CO2(g)
d) C(s) + O2(g) → CO2(g)
e) C(s) + CO2(g) → 2CO(g)
f) CaCO3(s) → CaO(s) + CO2(g)
g) CaO(s) + SiO2(s) → CaSiO3(l)
3. Balance the following equations by oxidation-reduction methods; note that three elements change oxidation state.
Co(NO3)2(s) →Co2 O3(s) + NO2(g) + O2(g)
4. How does the carbon-atom hybridization change when polyethylene is prepared from ethylene
5. Write the chemical formula, condensed formula, and Lewis structure for each of the following hydrocarbons:
(a) heptane
(b) 3-methylhexane
(c) trans-3-heptene
(d) 4-methyl-1-hexene
(e) 2-heptyne
(f) 3,4-dimethyl-1-pentyne
6.Write Lewis structures and describe the molecular geometry at each carbon atom in the following compounds:
(a) cis-3-hexene
(b) cis-1-chloro-2-bromoethene
(c) 2-pentyne
(d) trans-6-ethyl-7-methyl-2-octene
7.Write two complete, balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures.
(a) 2-butene reacts with chlorine.
(b) benzene burns in air.
8. Give the complete IUPAC name for each of the following compounds:
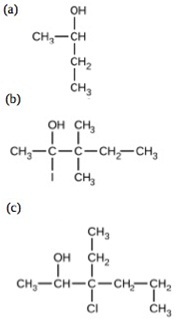
9.Draw the condensed formulas for each of the following compounds:
(a) dipropyl ether
(b) 2,2-dimethyl-3-hexanol
(c) 2-ethoxybutane
10. Write a condensed structural formula, such as CH3CH3, and describe the molecular geometry at each carbon atom.
(a) propene
(b) 1-butanol
(c) ethyl propyl ether
(d) cis-4-bromo-2-heptene
(e) 2,2,3-trimethylhexane
(f) formaldehyde
11.The foul odour of rancid butter is caused by butyric acid, CH3CH2CH2CO2H.
(a) Draw the Lewis structure and determine the oxidation number and hybridization for each carbon atom in the molecule.
(b) The esters formed from butyric acid are pleasant-smelling compounds found in fruits and used in perfumes. Draw the Lewis structure for the ester formed from the reaction of butyric acid with 2-propanol.
12. Write the Lewis structures of both isomers with the formula C2H7N.