Reference no: EM131355983
Consider a van der Waals gas contained in the apparatus described in Problem 3.4-1 (i.e., in the "constant volume gas thermometer").
a) Assuming it to be known in advance that the gas obeys a van der Waals equation of state, show that knowledge of two reference temperatures enables one to evaluate the van der Waals constants a and b.
b) Knowing the constants a and b, show that the apparatus can then be used as a thermometer, to measure any other temperature.
c) Show that knowledge of three reference temperatures enables one to determine whether a gas satisfies the van der Waals equation of state, and if it does, enables one to measure any other temperature.
Problem 3.4-1
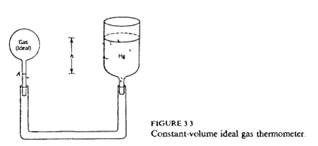
A "constant volume ideal gas thermometer" is constructed as shown (schematically) in Fig. 3.3. The bulb containing the gas is constructed of a material with a negligibly small coefficient of thermal expansion. The point A is a reference point marked on the stem of the bulb. The bulb is connected by a flexible tube to a reservoir of liquid mercury, open to the atmosphere. The mercury reservoir is raised or lowered until the mercury miniscus coincides with the reference point A. The height h of the mercury column is then read.
a) Show that the pressure of the gas is the sum of the external (atmospheric) pressure plus the height h of the mercury column multiplied by the weight per unit volume of mercury (as measured at the temperature of interest).
b) Using the equation of state of the ideal gas, explain how the temperature of the gas is then evaluated.
c) Describe a "constant pressure ideal gas thermometer" (in which a changing volume is directly measured at constant pressure).
|
How should state and local law enforcement executives
: There is a widespread perception among state and local law enforcement executives that reported crime will increase with the adoption of the National Incident Based Reporting System (N.I.B.R.S.), largely as a result of the elimination of the hiera..
|
|
What is the eight hour twa concentration
: What is the Eight hour TWA concentration in milligrams per cubic meter (mg/m3) - Think very carefully how you might do this. The final units are in mg/m3. You have mass in ug. You also have minutes and liters per minute. That should tell you how ma..
|
|
Responsibilities of a facilitator
: The homework: Explain the role and responsibilities of a facilitator. Identify a few "core values" for effective facilitators. Determine which ones are "most" important to you and your organization.
|
|
President of marketing for a hospital
: Imagine you are the vice president of marketing for a hospital that wants to implement a men's health product line. Write a 3-5 page assessment that answers the following:
|
|
Describe a constant pressure ideal gas thermometer
: Show that the pressure of the gas is the sum of the external (atmospheric) pressure plus the height h of the mercury column multiplied by the weight per unit volume of mercury (as measured at the temperature of interest).
|
|
Discuss motives for mass killings providing examples
: Discuss motives for mass killings providing examples.List two problems with current terrorism research and tracking.
|
|
Determine the factored moment capacity
: Determine the factored moment capacity ?Mn of the rectangular section subjected to interior environmental exposure with an initial deformation in the concrete substrate εbi = 0.0060.
|
|
Do the countries have a suitable infrastructure
: Do the countries have an acceptable economic, political, ethical, legal environment? Do the countries have a suitable infrastructure? Is one location in a country more suitable for the industry than another?
|
|
Ceo of a large healthcare system
: Take the perspective of the CEO of a large healthcare system that owns its own managed care health plan. Describe three major ways that you could improve the quality of healthcare in your organization.
|