Reference no: EM13983389
Free Energy and Chemical Thermodynamics Figure.
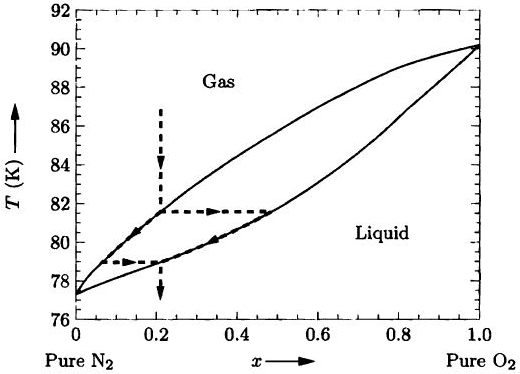
Experimental phase diagram for nitrogen and oxygen at atmospheric pressure. Data from International Critical Tables (volume 3), with endpoints adjusted to values in Lide (1994).
1. In this problem you will derive approximate formulas for the shapes of the phase boundary curves in diagrams such as Figures 5.31 and 5.32, assuming that both phases behave as ideal mixtures. For definiteness, suppose that the phases are liquid and gas.
(a) Show that in an ideal mixture of A and B, the chemical potential of species A can be written
μA = μo A + kT In(1 - x),
where μo A is the chemical potential of pure A (at the same temperature and pressure) and x = NB/(NA + NB). Derive a similar formula for the chemical potential of species B. Note that both formulas can be written for either the liquid phase or the gas phase.
(b) At any given temperature T, let xl and x9 be the compositions of the liquid and gas phases that are in equilibrium with each other. By setting the appropriate chemical potentials equal to each other, show that xl and xg obey the equations
(1-x1)/(1-xg) = eΔGoA/RT annd x1/ xg = eΔGoA/RT
where ΔGo represents the change in G for the pure substance undergoing the phase change at temperature T.
(c) Over a limited range of temperatures, we can often assume that the main temperature dependence of ΔGo = ΔHo - T ΔSo comes from the explicit T; both ΔHo and ΔSo are approximately constant. With this simplification, rewrite the results of part (b) entirely in terms of ΔHAo,ΔHBo, TA, and TB (eliminating ΔG and ΔS). Solve for xl and xg as functions of T.
(d) Plot your results for the nitrogen-oxygen system. The latent heats of the pure substances are ΔHoN2 = 5570 J/mol and ΔHoO2 = 6820 J/mol. Com-pare to the experimental diagram, Figure 5.31.
|
Why not compton scattering depend on properties of materials
: Why doesn't Compton scattering depend on properties of the materials that the electrons are in, the way that most other optical processes depend on specific properties of the material?
|
|
Differentiate the qualitative values of poetry and argue
: One of the goals for this unit is for you to be able "differentiate the qualitative values of poetry" and argue "why some works are timeless ‘masterpieces' and others have only short-term entertainment, emotional, or political value."
|
|
Analysis of the poem meaning or theme
: Write a 700- to 1,050-word explication of the poem. Note: An explication is a thorough analysis of the poem's meaning or theme supported by specific evidence from within the poem's text. Make sure you: Develop a thesis in paragraph one that clearl..
|
|
Describe the function of the committee
: Prepare this assignment according to the APA guidelines found in the APA Style Guide, located in the Student Success Center. An abstract is not required.
|
|
Derive a similar formula for chemical potential of species b
: Over a limited range of temperatures, we can often assume that the main temperature dependence of ΔGo = ΔHo - T ΔSo comes from the explicit T; both ΔHo and ΔSo are approximately constant. With this simplification, rewrite the results of part (b) enti..
|
|
Police community relations programs
: Why did early police community relations programs focus on minority communities?
|
|
Journal reflection paper on christianity
: Journal reflection paper on Christianity. The journal is to be an example of the student's ability to write and analyze the material he or she is reading
|
|
What is the final pressure of the ethane in the flask
: The stopcock is then closed, and the flask cooled to its original temperature. What is the final pressure of the ethane in the flask? Find the mass of ethane remaining in the flask.
|
|
Write a short story about restaurant
: The stabbing of a innocent woman: Write a 1 page short story about: Restaurant, 30 year old man, 32 year old woman, stabbing
|