Reference no: EM132358284
Specialised Topics in Chemistry Assignment -
Organometallic Chemistry Workshop -
Valence Bond Theory -
(1) What are the valence bond theory (VBT) hybridization schemes used to account for the following structures - (a) tetrahedral, (b) square planar and (c) octahedral?
(2) Experiments show that an cobalt(III) complex [Co(NH3)6]3+ is octahedral and there are no unpaired electrons. Explain this result using VBT? (Co, Z = 27).
(3) What is the VBT description of the above cobalt(III) complex if there are unpaired electrons?
Molecular Orbital Theory -
(4) (a) For the first-row transition metals, what are the valence atomic orbitals used for constructing a MO diagram for an octahedral metal complex?
(b) Construct a qualitative MO diagram for [Co(NH3)6]3+, for which there is no π-bonding between the metal and the ligands. Label the diagram with the appropriate atomic orbitals for the metal, and labels the resulting MO's as either bonding, antibonding or non-bonding and add the correct number of electrons from the metal and ligands to the diagram.
(c) Label Δo on the MO diagram.
(d) What type of orbitals (bonding, non-bonding, antibonding) are the "crystal field" orbitals?
Metal- Ligand π-bonding
(5) (a) Label each of the following, with the type of metal-ligand interaction (σ-interaction, π-donor or π- acceptor).
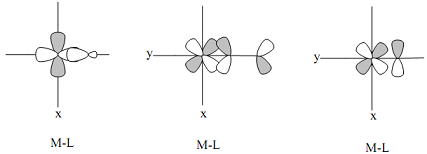
(b) In both the π-donor and π-acceptor cases, which set of metal orbitals (use symmetry label) are interacting with the ligand orbitals to form π-bonds?
(c) What is the difference between the π-donor and π-acceptor cases?
(6) Below are partial MO diagrams for metal-ligand π-bonding in octahedral complexes. The orbitals responsible for σ-bonding are not shown.
(a) Determine which diagram is for the π-donor and the π-acceptor cases.
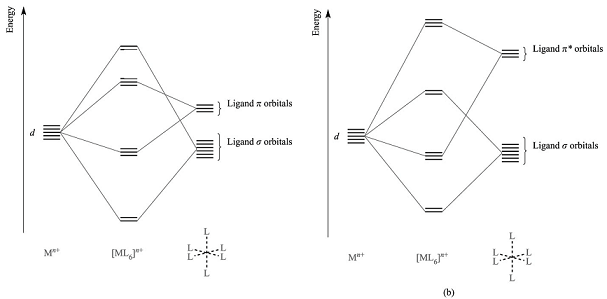
(b) What happens to the Δo in going from a complex with σ-bonding only to π-donor ligands?
(c) What happens to Δo in going from a complex with σ-bonding only to π-acceptor ligands?
(7) Give an example of a ligand for each of the three types of bonding in coordination complexes σ-only, π-donor and π-acceptor ligands).
(8) What relationship can you see between the arrangement of ligands in the spectrochemical series and the type of bonding that occurs between the ligand and the metal? Are the ligand examples you gave in question (7) consistent with the positions of these ligands in the spectrochemical series?
Organometallic Chemistry Problem Set -
Revision -
(1) Give definitions of the terms shielding and effective nuclear charge as applied to atoms.
Slater's Rules
Zeff = Z - S
Zeff = Effective Nuclear Charge
Z = Nuclear Charge
S = Screening or Shielding Constant
To determine S (shielding constant)
1. Write out the electronic configuration of the element in the following order and groupings: (1s), (2s, 2p), (3s, 3p), (3d), (4s, 4p), (4d), (4f), (5s, 5p) etc.
2. Electrons in any group higher in this sequence than the electron under consideration do not shield those in lower groups and contribute nothing to S.
3. Consider a particular electron in an ns or np orbital:
(i) Each of the other electrons in the (ns, np) group contribute S = 0.35.
(ii) Each of the electrons in the (n - 1) shell contribute S = 0.85.
(iii) Each of the electrons in the (n - 2) group contribute S = 1.0.
4. Consider a particular electron in an nd or nf orbital:
(i) Each of the other electrons in the (nd, nf) group contribute S = 0.35
(ii) Each of the electrons in a lower group than the one being considered contribute S = 1.0
(2) Using Slaters rules confirm that the experimentally observed electronic configuration of K, 1s1 2s2 2p6 3s2 3p6 4s1 is energetically more stable than the configuration 1s1 2s2 2p6 3s2 3p6 3d1.
(3) Calculate the value of Zeff for a 2p electron of F (Z=9).
(4) Use Slater's rules to estimate values of Zeff for (a) a 4s electron and (b) a 3d electron in a Vanadium atom (Z=23).
Ligands and Crystal Field Theory -
(5) Draw the structures of the following ligands, highlight the donor atoms and give the likely modes of bonding (e.g. monodentate): (a) en; (b) bpy; (c) [CN]-, (d) [N3]-; (e) CO; (f) phen; (e) [Ox]2-; (h) [NCS]-.
(6) Outline how you would apply crystal field theory to explain why the five d-orbitals in an octahedral complex are not degenerate. Include in your answer an explanation of the 'barycentre'.
(7) Arrange the following ligands in order of increasing crystal field strength: Br-, F-, [CN]-, NH3, [OH]-, H2O.
(8) For which member of the following pairs of complexes would Δoct, be the larger and why: (a) [Cr(OH2)6]2+ and [Cr(OH2)6]3+; (b) [CrF6]3- and [Cr(NH3)6]3+; (c) [Fe(CN)6]4- and [Fe(CN)6]3-;( d) [Ni(OH2)6]2+ and [Ni(en)3]2+; (e ) [MnF6]2- and [ReF6]2-; (f) [Co(en)3]3+ and [Rh(en)3]3+.
Valence Bond Theory problems -
(9) Experiments show that an Iron(II) complex is octahedral and there are no unpaired electrons. What is the valence bond theory description?
(10) What is the VBT description of the above complex if there are unpaired electrons?
(11) Experiments show that a nickel(II) complex is square planar and there are no unpaired electrons what is the VBT description?
(12) Experiments show that a nickel(II) complex is tetrahedral and there are two unpaired electrons what is the VBT description?