Reference no: EM13972986
1. Poly(ε-caprolactone) is being considered as a potential material for a vascular graft. After a particular batch has been made, someone gives you the following fractional distribution data and asks you to calculate Mn, Mw, and polydispersity index (PDI) for this polymer:
Wi(kg) 0.1 0.1 0.3 0.4 0.3
Mi (kg/mol) 25 35 45 70 100
What are the weight and mole fractions of the polymeric fraction with molecular weight of 70.
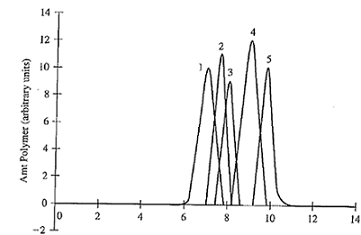
2. You are given three polymeric materials (A,B,C) to examine for potential as a vascular graft material, and you are told to use size-exclusion chromatography to determine the approximate molecular weights of the unknown polymers. You obtain the following information for the three samples (A,B,C):
Assuming monodisperse samples, what is the molecular weight of each knowing that:
Standard 1: Molecular weight 50,000 g/mol
Standard 2: Molecular weight 40,000 g/mol
Standard 3: Molecular weight 35,000 g/mol
Standard 4: Molecular weight 20,000 g/mol
Standard 5: Molecular weight 10,000 g/mol
Unknown A: Peak amount polymer eluted at 7.4 min
Unknown B: Peak amount polymer eluted at 8.4 min
Unknown C: Peak amount polymer eluted at 9.6 min
3. The rate of a step polymerization reaction between monomers A and B is given by the following expression:-d[M]/dt = k*[M]4
where [M] describes the concentration of the unreacted functional (reactive) groups and k is the rate constant of the polymerization reaction. Calculate the monomer concentration [M], degree of polymerization Xn and the extent of conversion p after 25 hours of reaction. The concentrations of the functional groups are at stoichiometric ratio and the initial monomer concentration [M]o is 15 moles/L. The rate constant k is equal to 0.2 L3/(min*moles3).
4. a) The number average molecular weight of polyacrylonitrileis 1,000,000 g/mol. Compute the number-average degree of polymerization.
b) Determine the molecular weight of the following polymers:
- Polyethylene, a material commonly used in the wearing surface of acetabular cup component of hip prostheses: -(CH2-CH2)100-
- Polymethylmethacrylate, a material commonly used as a bone cement in orthopedic procedures: -[-CH2-(CH3)C(CO2CH3)-]150-
- Polyvinyl alcohol: -[CH2-CH(OH)-]300-
5. Select a polymer prepared under a step or chain polymerization reaction that is used in a biomedical application (i.e. prosthetics, drug delivery, dressings etc.). Perform a literature search on this polymer and write a paragraph describing:
a. The reaction conditions (i.e. catalysts use, processing techniques, etc.)
b. The biomedical application where the polymer is used and the performance specifications (i.e. mechanical characteristics, biocompatibility, degradation, etc.) that the polymer has to fulfill in its use.
6. The molecular weight distribution for polyethylene is given in the following table:
Mi (g/mol) xi Wi
8,000 (A) 0.167 16,000
12,000 (B) 0.250 36,000
20,000 (C) 0.333 80,000
30,000 (D) 0.250 -
Calculate the number average molecular weight, weight average molecular weight and the polydispersity of the polymer. xi and Wi denote the molar fraction and the weight of the ith species with molecular weight of Mi respectively.
7. A strip of tissue was excised for mechanical testing in tension. The initial dimensions of the rectangular specimen were 30 mm long and 15 mm wide, with an average thickness of 3 mm. The mechanical testing was conducted at a rate of 5 m/sec. the following data was obtained:
Gauge length (mm) Force (N)
20 0
20.5 0.1
21 0.3
21.5 0.5
22 0.8
22.5 1.1
23.1 1.6
23.6 2.0
24.2 2.7
25.2 4.7
26.3 7.9
27.4 11.4
27.9 12.9
28.5 14.5
29.0 16.4
30.1 19.6
a) Calculate the engineering stresses and strains from the information given and plot the engineering stress-strain curve. Assume that 5 mm of the specimen length is clamped by the testing grips at each end, such that the initial gauge length of the specimen is 20 mm.
b) It was found that immediately before the last data point, the average width of the sample was 8 mm and the average thickness of the sample was 0.75 mm. Considering this information determine the true stress and true strain of the sample at the last data point.