Reference no: EM131111115
Atoms and molecules
1. The diagram shows the atomic structure of an atom of element X.
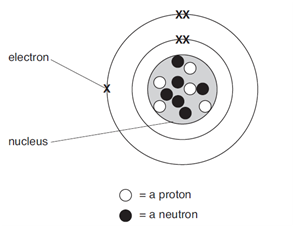
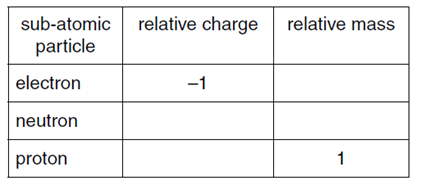
(a) Complete the table.
(b) Carbon-12 has the symbol 126C.
Write the symbol for an atom of element X...............................................................................................
2. The diagram shows the nuclei of five different atoms.

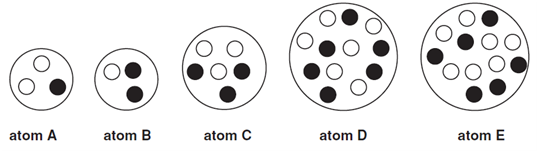
(a) Which atom has an atomic number of ....................................................................................................
(b) Which atom has a mass number of 6?....................................................................................................
(c) Which two atoms are isotopes of the same element?..................................................... and ......................................................
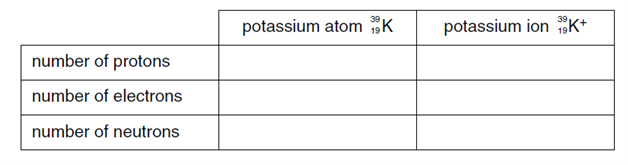
3.a) Complete the table below to show the number of sub-atomic particles in both an atom and an ion of potassium.
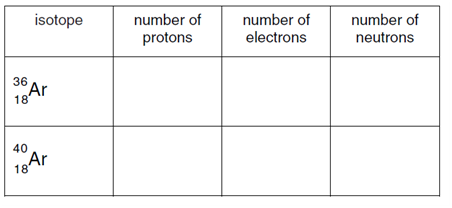
b) Complete the table to show the number of particles in two isotopes of argon.
4. These diagrams show the electron arrangement in the outer shells of five elements, A to E.
All elements are from Period 3 of the Periodic Table.
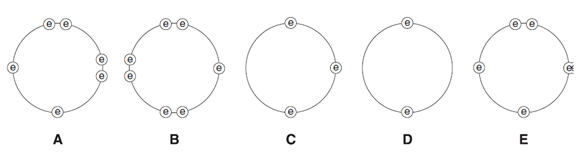
(a) Put the letters A to E in the table to show which elements are metals and which are nonmetals.

(b) Which element is most likely to be in Group VI?................................................................................................................................
(c) Which element will form an ion of the type X2+ ? ......................................................................................................
(d) Which element has an atomic number of 15? ....................................................................................................
(e) Which two elements will form an ionic compound with a formula of the type YZ2? ..........................................................................................
5. (a) Define the term element.
....................................................................................................................................
....................................................................................................................................
(b) Choose from the following elements to answer the questions below.
Aluminium argon bromine gallium helium
Hydrogen Magnesium nitrogen oxygen sodium
Each element can be used once, more than once or not at all.
Which element
(i) is in Group III and Period 4 of the Periodic Table, ...............................................
(ii) has atoms with 8 electrons in their outer shell, ...................................................
(iii) is a liquid at room temperature, ........................................................................
(iv) reduces unsaturated vegetable oils to form a solid product, ............................
(v) forms an ionic chloride with the formula XCl2, ....................................................
(vi) is used in light bulbs? ..........................................................................................
6. The diagram shows part of the Periodic Table.
Answer these questions using only the elements shown in the diagram.
Each element can be used once, more than once or not at all.
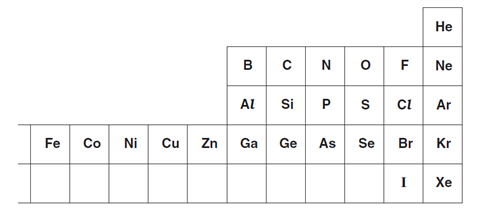
Write the symbol for
(i) an element which is in Group 5 and Period 3,.................................
(ii) an element which is used as a gas in balloons, .................................
(iii) an element which forms ions in aqueous solution which give a white precipitate on reaction with aqueous silver nitrate, .................................
(iv) an element which forms an ion of type X 3- .................................
(v) an element which is a catalyst for the hydrogenation of alkenes .........................................
(vi) two elements which combine to form a compound which causes acid rain. .................................