Reference no: EM132503899
Question 1. What is the order of reaction for a reaction that has the following stoichiometric equation.
A + B → 2 C.
Question 2. The rate expression for reaction
2 A + 0.5 B 0.5 C + D is given as
-rA = 100C2ACB0.5.
If the reaction stoichiometric equation is written as 4A + B → C + 2D
what would be the rate expression?
Question 3. Free radical polymerization of A takes place as per following reaction in a 2 dm3 mixed flow reactor:
A → B → C → D .....
Stream of aqueous monomer A (1 mol/dm3, 4 dmˆ3/min) is fed into the reactor and where it undergoes polymerization. In the exit stream CA = 0.01 mol/dm3, and for a particular reaction product J, CJ = 0.0002 mol/dm3. Find the rate of reaction of A and the rate of formation of J.
Question 4. Glyphosate dissolved in water (concentration: 1 mol/dm3) is degraded in two aerobic stirred reactors arranged in series. The concentration of glyphosate is reduced to half at the end of the first aerobic tank. Find the glyphosate concentration in the exit stream of the second tank. The reaction is second-order with respect to glyphosate and the ratio of the volume of second tank to the volume of first tank is 2.
Question 5. Example 5-4 Calculating Pressure Drop in a Packed Bed: Pressure drop in a packed bed reactor is given by
P/P0 = (1-2βL0/P0)1/2
β = G(1-Φ)/gcρdpΦ3 [150 (1-Φ)μ/dp + 1.75 G]
G = m·/Ac
1. The reactor was operated in a turbulent regime. How would the pressure drop parameter
β) change if the particle diameter was reduced by 25%?
2. Can you determine when "no flow" condition will occur?
Question 6. Fairy floss maker: We are prototyping a 1 dm3 fairy floss maker to be operated in steady flow. First tests in this unit show that 1 dm3/min of raw sugar stream produces 28 dm3/min of mixed exit stream. Controlled experiments have shown the volumetric expansion of sugar particles to be 31 times (1 dm3 raw sugar makes 31 dm3 fairy floss, [A → 31 B]). With this information determine what fraction of raw sugar is converted in the unit.
Question 7. In an isothermal batch reactor 70% of a liquid reactant is converted in 13 min. What space-time and space-velocity are needed to effect this conversion in a plug flow reactor and in a mixed flow reactor?
Question 8. In order to achieve 82% conversion, what order of reactors will lead to the minimum reactor volume? Explain with a schematic.
Reactor 1: Small PFR
Reactor 2: Large PFR
Reactor 3: CSTR
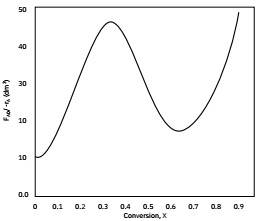
Question 9
A new drug, Covium, is found particularly effective against COVID-19. Covium is formed by a reaction of newlium and oldium as per following reaction.
N + O ↔ C
The reaction rate has been measured from previous laboratory studies and has been found to be elementary.
Before full scale manufacture is possible a pilot scale run of this liquid phase reaction must be made. We currently have available two stirred tank reactors with volumes of 50 m3 and 500 m3.
The reaction rate constants at the recommended reaction temperature of 162 K are kf = = 2 × 10-3 m3/mols and kreverse = 5 × 10-4 1/8 .
As in most pharmaceutical operations, the reaction is conducted in batches to guarantee a sterile product. The initial concentrations of newlium and oldium are each 0.5 mol/m3 .
1. Construct a Stoichiometric table.
2. Determine the maximum conversion of newlium that can be obtained in this batch reactor.
3. Determine the time required to obtain the conversion found in part b.
4.Which reactor would you use for this pilot plant run? Give an explanation.
Question 10
The homogeneous gas phase reaction, A → 3C, takes place in a plug flow reactor at 215 °C. The rate equation at this temperature is given as -rA = 10-2CA1/2 [mol/dm3.s]
A mixture of 50% A and 50% inert is fed into the reactor. The inlet concentration of A is 0.0625 mol/dm3. The reactor is operated at 5 atm pressure and the pressure drop across the reactor is negligible. Find the space-time needed for 80% conversion. (Hint: You can use either graphical or numerical integration method.)