Reference no: EM132854868
CHEM3580 Colloids, Interfaces and Soft Matter - The University of Newcastle
Question 1. Draw a sketch of a negatively charged particle in a solution of a 1:1 electrolyte, indicating the position of counterions and co-ions around the particle. Indicate the position of the shear plane.
(b) A 500 nm titania particle has an electrophoretic velocity of 160 μm/s in a 4000 V/m electric field in a 0.01 M solution of NaBr at 25 °C (fluid density = 1000 kg/m3). Calculate:
(i) The particle mobility UE
(ii) The particle zeta potential
Question 2. The DLVO total potential energy of interaction between two negatively charged colloidal particles is given in the figure below for five different surface potentials (proportional to surface charge density, σ). Discuss the colloidal stability of the particulate dispersion under each of the five conditions illustrated.
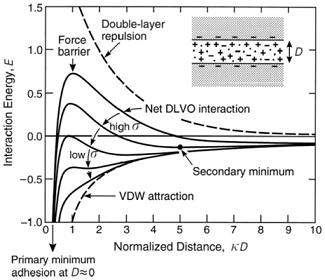
Question 3. A stable colloidal dispersion of gold particles (radius = 10 nm) has the appearance of a bright red translucent suspension. Addition of electrolyte is seen to result, at some critical concentration, in the formation of an aggregated system. The onset of coagulation is observed by a change in the system to a dark purple colour. The following coagulation concentration was recorded for NaCl = 55 mM.
(a) What characteristics of the gold colloids gives rise to the original system colloidal stability?
(b) What is the role of electrolyte in altering this stability?
(c) Estimate the concentration of the following electrolytes that could be used as alternative coagulants: KCl, CaCl2 or AlCl3. Discuss.
Question 4. The following graph shows the zeta potential of alumina (Al2O3) and zirconia (ZrO2, YSZ) colloidal dispersions as a function of pH both in the absence, and presence of the polymeric dispersant D-3005. Using the data in the graph address the following:
(a) What is the point of zero charge of the two minerals: alumina and zirconia?
(b) What is the likely dominant surface functional group on each mineral at pH 4, and at pH 10?
(c) Briefly discuss the pH-dependent colloidal stability of both minerals.
(d) Discuss the role of the soluble polymeric dispersant on the dispersion stability of each mineral. What are the likely characteristics of this polymer? What is the nature of the polymer-mineral interaction?
Attachment:- Interfaces and Soft Matter.rar
|
Levels of customer service
: Has the philosophy that there are different levels of customers had any material effect on the value of your organization's customer service?
|
|
Find the smallest sample size
: If three sigma limits are used, find the smallest sample size that would yield a positive lower control limit.
|
|
Forecasting approaches to logistics within the supply chain
: Has the current economic environment changed your organization's forecasting approaches to logistics within the supply chain?
|
|
Explain how social determinants of health for older adult
: Explain How social determinants of health for the older adult are impacted for those living in poverty. Healthy aging is an important public health issue.
|
|
CHEM3580 Colloids, Interfaces and Soft Matter Assignment
: CHEM3580 Colloids, Interfaces and Soft Matter Assignment Help and Solution, The University of Newcastle - Assessment Writing Service
|
|
Sketch a graph of the distribution
: Sketch a graph of the distribution, including the height to make this a proper density.
|
|
Implementation-strategic controls and contingency plans
: Write a draft of about of the strategic plan for your organization, Key success factors, budget, and forecasted financials, including a break-even chart
|
|
How are health care needs within the school district
: Do the schools in your area have a school health nurse assigned? If not, how are health care needs within the school district managed?
|
|
Differences between null and alternative hypotheses
: What is the difference between failing to reject the null hypothesis and having evidence to support the alternative hypothesis?
|