Reference no: EM132463778
CHEM1110 - Drug Discovery & Medicinal Chemistry - University of Greenwich
Question 1. Enalaprilat (shown below) is an acetylcholinesterase (ACE) inhibitor. The structure is reproduced several time below. On each structure, highlight the function(s) specified but circling the appropriate atom/group of atoms.
a) label/circle all the acid and basic sites, clearly indicating which is which.
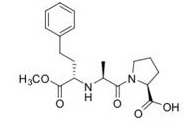
b) label/circle all the hydrogen bond donors (HBD) and hydrogen bond acceptors (HBA), clearly labelling which is which.
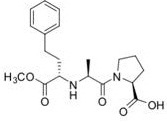
c) label all the sites which could potentially interact with a receptor, and clearly indicate the type of interaction that would be involved (e.g. hydrogen bonding, ionic interactions)
d) label four possible sites of primary metabolism, clearly indicating the type of reaction/transformation at each site.
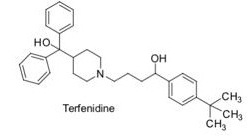
Question 2. All antihistamines (histamine antagonists) act on histamine H2 receptors throughout the body. Older ones, such as diphenhydramine are known to have sedative side effects, through antagonism of HI receptors, only found in the brain. Newer histamine antagonists, such as terfenadine and cimetidine do not show similar sedative side effects. Suggest reasons why these show no such side effects.
Question 3. One of the compounds in Q2, Terfenidine, was a widely used anti-histamine until its withdrawal from the market in the 1990's, owing to its interaction with the hERG receptor.
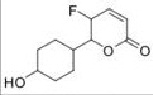
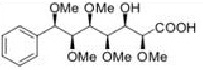
Question 4. Assess the following compounds for their compatibility with Lipinski's guidelines. Recommend their overall feasibility as drug candidates
|
|

Concentration in octanol - 18.7 gcm-3
Concentration in water = 13.9 gcm-3
|
|
Molecular formula =
Molecular weight =
Hydrogen bond donors - Hydrogen bond acceptors=
Log P =
|
|
Molecular formula =
Molecular weight =
Hydrogen bond donors =
Hydrogen bond acceptors=
Log P =
|
|
|
Recommendation: ..............
|
Recommendation: ..........
|
|
Concentration in octanol = 4.6 gem.'
Concentration in water = 22.3 pm')
|
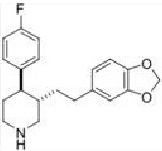
|
|
Molecular formula =
Molecular weight =
Hydrogen bond donors =
Hydrogen bond acceptors=
Log P =
|
|
Molecular formula =
Molecular weight =
Hydrogen bond donors =
Hydrogen bond acceptors=
|
|
|
Recommendation:...............
|
|
Recommendation: ............................
|
|
|
|
|
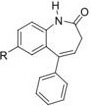
Question 5. A QSAR analysis of the effects of substituents R on the anticonvulsant activity gave the following Hansch equation:
|
log (1/C)= 0.19[] + 1.20[] + 4.56
n = 10
|
|
|
|
|
|
|
NH2 |
OH
|
CN
|
NO,
|
H
|
F
|
SO2PH
|
OEt
|
CH3
|
Cl
|
CF3
|
NEt2
|
|
[]:
|
-1.23 |
-0.67
|
-0.57.
|
-0.28
|
0
|
0.14
|
0.27
|
0.38
|
0.56
|
0.71
|
0.88
|
1.18
|
|
[]:
|
-0.66 |
-0.37
|
0.66
|
0.78
|
0
|
0.06
|
0.7
|
-0.24
|
-0.17
|
0.23
|
0.54
|
-0.90
|
a) Define the terms __ and __.
b) comment on the value of n:
c) From the table, which substituent would be the best choice to give optimal activity? Briefly justify your answer.
d) Circle, on the structure below, three possible sites of Phase 1 and Phase 2 metabolism. Clearly indicate the transformation that you are suggesting will occur at that position.
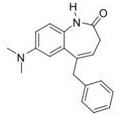
Question 6. The measured log P values for five aromatic compounds are shown below.
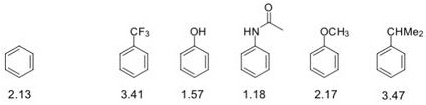
a) Given that log P for benzene is 2.13, calculate the hydrophobic parameter (9) for each of the six substituents, and hence classify the substituents as hydrophilic or hydrophobic. Show your working.
b) Using your answers from part (i), calculate the log P values for the following two molecules.
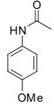
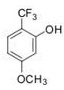
Question 7. Based on the Craig plot provided:
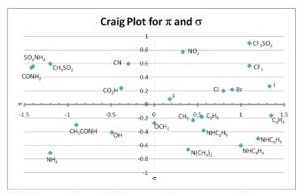
i) which substituent has a small electron donating effect and maximizes hydrophilicity?
ii) which substituent has the largest electron donating effect?
iii) A series of phenols gives the following Hansch equation:
 log(1/C) = 1.2Π - 0.7σ + 3.1
log(1/C) = 1.2Π - 0.7σ + 3.1
i) What type of properties should the R group possess to maximise biological activity?
ii) Use the Craig plot to predict several likely candidates to prepare for study and briefly explain your reasoning:
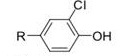
Question 8. In 2004, a group of medicinal chemists from Celltech (Cambridge, UK), published a paper describing the synthesis of a series of potent and selective IKK2 inhibitors (Davenport et al,Bioorg. Med. Chem. Lett., 2004. 14,409-412).
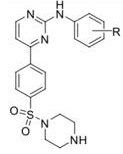
a) In this paper, what do they state, in terms of Hammett __ and Hansch _ values, gave the greatest activity?
b) What were the values of a 0 and log P for compounds where R =
i) R = 4-OCH3 ii) R = 4-CN iii) R = 4-COCH3
[]= []= []=
[]= []= []=
log P = log P = log P =
Question 9. Molecule (1) shown below is a synthetic drug developed from the mitomycin family of anti-cancer agents.
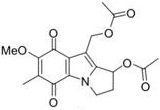
1. Does (1) conform to the Lipinski 'Rule of 5'? Explain your answer.
2. (1) does not react directly with DNA. What transformation must it first undergo before it can form DNA adducts? Give the structure of the neutral product arising from the initial transformation.
3. Propose an outline reaction mechanism to explain how (1) may form a DNA interstrand crosslink. [Your answer should include some curly arrows and an indication of how it interacts with (which bits of) DNA].
Question 10. The structures of dihydrofolate reductase bound with inhibitor/anti-cancer drug Methotrexate have been obtained from crystallographic study. One of these structures is available from the protein data bank with code 4GH8.pdb.
You should utilise the visualisation program implemented in the website of protein data bank to produce two pictures. Your coursework should contain (1) a picture to show the overall structure of the enzyme (dihydrofolate reductase), displayed in its secondary structure (2) a picture to show, with atomistic detail, how Methotrexate interacts with the active site of the enzyme in particular the side chain of residue Arg61. In your picture 2, you should also display the distance between atom CT of Methotrexate and one of the nitrogens of the guanidinium group of Arg61.