Reference no: EM132551609
Case Study of Absorption Process for Developing and Analyzing Process Dynamics
The removal of sulfur dioxide (SO2) from combustion gas is required for environment safety. This can be achieved by gas- liquid absorption using a liquid absorbent. This is a multi-stage process. A simplistic example is given below with only two stage absorption of SO2.
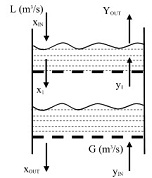
The liquid absorbent goes down while the combustion gas moves up through the perforated sieve trays. To maintain a significant liquid hold-up on each stage weirs and down comers are used. While, a significant pressure drop is used to force the gas to flow upward through the perforations. The upward moving gas comes into contact with the liquid absorbent. We can assume that the component to be absorbed is in equilibrium between the gas and liquid streams leaving each stage i due to the intimate mixing. Assume a simple linear relation between the gas concentrations of the absorbed component, yi, leaving a given tray i and the liquid concentration of the absorbed component xi at the given tray i
yi = axi + b (A)
Note: concentrations here are in terms of kg/m3
Assume constant liquid holdup AH (area × height) and perfect mixing on each stage, and neglect any hold-up of gas (dyi/dt = 0 and dyout/dt = 0), develop dynamic model for the given simplistic system of two trays.
Q 1.1 Develop dynamic model for the concentrations of absorbed SO2 for the two stages to obtain dxi/dt = 0 and dxout/dt = 0 by applying mass conservation principle on absorbed SO2.
(Hint: Consider the SO2 in both gas and liquid.)
Simplify the above model by using equilibrium relation given by Eqn. (A). Obtain the dynamic model in terms of deviation variables.
Q 1.2 Assume Xin as constant (deviation variable XIN = 0) and substitute τ1 = AH/L= 2 min (the stage liquid residence time), F = aG/L= 1 (the stripping factor), R = G/L=2. Obtain the transfer function Yout(s)/Yin(S) by simplifying the above two dynamic models.
Q 1.3 Suppose that there is a step change in SO2 concentration of the combustion gas with 5 units at inlet. Draw the diagram of the response of the absorption column in form of the SO2 concentration of the combustion gas leaving the column. Find out the maximum variation in the response. How it is affected by the process parameters
Attachment:- Problem on Process Dynamics.rar