Reference no: EM13910158
Problem 1:
(a) E.coli swims at about 20 p.111/S by rotating a bundle of helical flagella. If the motors were to turn 10 times faster than normal, what would their swimming speed be? If their fluid environment were made 10 times more viscous, but the motors were to turn at the same rate, what would the swimming speed be? How does the power output of the motor change in these two hypothetical situations?
(b) Two micron-sized spheres, one made of silver and the other gold, sediment (that is, fall under gravity) in a viscous fluid. The silver sphere has twice the radius of the gold one. Which sediments faster?
(c) The left ventricle of the human heart expels about 50 cm3 of blood per heartbeat. Assuming a pulse rate of 1 heartbeat per second and a diameter of the aorta of about 2 cm, what is the mean velocity of blood in the aorta? What is the Reynolds number?
(d) What is the Reynolds number of a swimming bacterium? A tadpole? A blue whale? (Adapted from a problem courtesy of H. C. Berg and D. Nelson.)
Problem 2:
Show that the Boltzmann factor for a system in a state with degeneracies i∑e-Ei/kBT
where i is a sum over degenerate states of the same energy can be written as e-Fi/kBT. In the same way, when dealing with constant pressure, the Boltzmann factor becomes e-Gi/kBT . This is what you need in problem no. 7.
Problem 3:
Particles in suspension in water have a mean height distribution of zi *= 2 cm. To produce a colloidal suspension that does not settle at the bottom of a jar of 10cm height, the particles need to be broken up into smaller pieces. In how many equal fragments should these particles be broken so that z2* = 10 cm?
Problem 4:
A protein has two conformations, compact (C) and expanded (EX) with an energy difference of 3kbTR, the lower energy one C has a degeneracy gc = 2. The higher one EX has a degeneracy gEx.
Determine the value of gEx such that at room temperature C is ten times as likely as EX.
This protein is placed in a lipid bilayer, where its configuration is sensitive to the tension in the bilayer. The two states have areas Ac = 10 nm2 and AEx = 20 nm2 respectively. At what tension will the probability of occupancy of the two states be equal, recalling that under a constant tension the Gibbs free energy will be minimized G = U - TS - TA (T is an applied tension)? Take gEx = 5 for this part.
Problem 5:
To add to your insight on the partition function, show that Z = Ωexp(-β<E>), where ,Ω is the number of states and< E> the average energy for a particular choice of thermodynamic variables.
Problem 6:
Helix-coil transition (from Nelson no. 9.5 p. 396)
We will explore a simple model of the helix-coil transition in an artificial polypeptide (see Zimm et al PNAS 45:1601-1607 (1959)). The helix is an ordered molecule which has higher energy than the random coil version, but still as the temperature is raised the transition goes from random coil to helix. The following simple model of "total cooperativity" provides a simple explanation. "total cooperativity" simply means that the each molecule is either a random coil or a helix and not segments of each of the two phases.
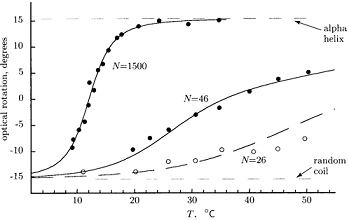
(i) In the optical rotation experiment shown above, we see a continuous variation of the rotation angle of polarized light and not a jump from the value associated with the random coil to the value associated with the helix. Explain qualitatively why.
(ii) Now calculate the proportion of molecules in the helix phase as a function of temperature.
|
Question regarding the final volume
: A gas with an initial volume of 24.0 L at a pressure of 565 mmHg is compressed by a pressure increase of 0.25 Pa. What is the final volume (L) of the gas, assuming the temperature does not change?
|
|
Partial pressure of argon twice
: A flask contains 2.00 moles of nitrogen and 2.00 moles of helium. How many grams of argon must be pumped into the flask in order to make the partial pressure of argon twice that of helium?
|
|
Define money and its different types
: Evaluate current financial markets according to their ability to do their function? Hint: explain the function of financial market and give an opinion whether the current financial markets are able to fulfill these functions?
|
|
Commercial hog farm operation
: What will dissolve uric acid crystallizations clogging pvc pipes in a commercial hog farm operation? Surfactant must be non dangerous to the water table as pig effluents are drained into a lagoon.
|
|
Calculate the proportion of molecules in the helix phase
: Determine the value of gEx such that at room temperature C is ten times as likely as EX - calculate the proportion of molecules in the helix phase as a function of temperature.
|
|
How many times a year will the firm turn over its inventory
: Assume that a firm has a payables deferral period of 40 days, an inventory conversion period of 62 days, and an average collection period of 29 days. What's the firm's cash conversion cycle?
|
|
Why do venture capitalists buy convertible preferred stock
: ABC is considering increasing its dividend payout to shareholders in the future and therefore plans to raise debt this year. If the company issues additional debt of £20 million, what would be the effect on the firm's WACC? Please explain your res..
|
|
Analysis of the income statements
: 1.Horizontal and vertical analysis of the Income Statements for the past three years (all yearly balances set as a percentage of total revenues for that year).2.Horizontal and vertical analysis of the Balance Sheets for the past three years (all y..
|
|
Acid base chemical equation
: Hydrochloric acid is an important commercial acid. Hydrochloric acid reacts with ammonium hydroxide forming ammonium chloride. (i) Write the acid base chemical equation for above reaction.
|