Reference no: EM13884559
1. (a) Determine the amount of heat (in kJ) given off when 1.26 ×104 g of ammonia are produced from the following reaction:
N2 (g) + 3H2(g) → 2NH3(g); ?H°rxn = -92.6 kJ/mol
that the reaction takes place under standard conditions at 25°C.
(b) A 12.1 g piece of aluminum with a temperature of 81.7°C is added to a sample of water at 23.4°C in a constant-pressure calorimeter of negligible heat capacity. If the final temperature of water is 24.9°C, calculate the mass of the water in the calorimeter.
(c) Ethylene, C2H4, a compound with a carbon-carbon double bond, add hydrogen in a reaction called hydrogenation.
C2H4 (g) + H2(g) → C2H6(g)
Calculate the enthalpy change for this reaction, using the following combustion
data:
C2H4 (g) + 3O2(g) → 2CO2(g) + 2H2O(l); ?H = -1411 kJ
C2H6 (g) + 7/2O2(g) → 2CO2(g) + 3H2O(l); ?H = -1560 kJ
H2 (g) + 1/2O2(g) → H2O(l); ?H = -286 kJ
2. (a) Calculate the partial pressures of helium and neon after the stopcock of the following apparatus is open. Assume that the temperature remains constant at 16°C.
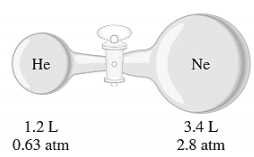
(b) The pressure of 6.0 L of an ideal gas in a flexible container is decreased to onethird of its original pressure, and its absolute temperature is decreased by onehalf. What is the final volume of the gas?
(c) Calculate the mass of water vaporized if 10.00 g of water is introduced into an evacuated flask of volume 2.500 L at 65°C. Assume that the volume of the remaining liquid water is negligible and the vapor pressure of water at 65°C is 187.5 mmHg.
3. (a) Sketch the phase diagram of argon, Ar, from the following information:
Normal melting point = -187°C
Normal boiling point = ?186°C
Triple point = ?189°C, 0.68 atm
Critical point = ?122°C, 48 atm
Label the triple point, critical point and each phase region in the diagram.
3(b) Which substance in each of the following pairs would you expect to have the higher boiling point? Explain why.
(i) Ne or Xe
(ii) CO2 or CS2
(iii) CH4 or Cl2
(iv) F2 or LiF
(v) NH3 or PH3
(c) Barium metal has a body-centered cubic lattice with all atoms at lattice points. Its density is 3.51 g/cm3 .From these data and the atomic weight, calculate the edge length of a unit cell.
4. (a) Calculate the valence electrons and draw complete Lewis structures, including lone pairs, for the following condensed structures:
(i) CH2CHCN
(ii) (HOCH2)2CO
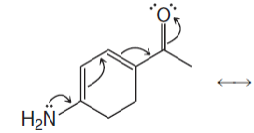
(b) Write the resonance structure that would result from moving the electrons in the way indicated by the curved arrows:
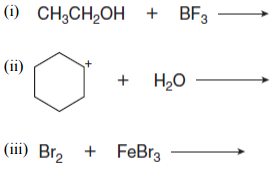
(c) Draw the products of each Lewis acid-base reaction. Use curved arrows to show the movement of electrons in each reaction. Label the electrophile and nucleophile.
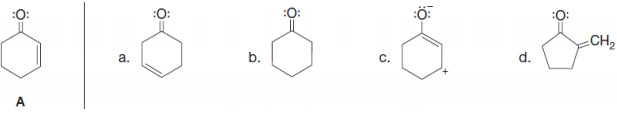
5. (a) By using compound A as a reference, label each compound as an isomer, a resonance structure, or neither. Explain your answer.
(b) Ketene, CH2=C=O, is an unusual organic molecule that has a single carbon atom doubly bonded to two different atoms. Determine the hybridization of both C atoms and the O in ketene. Then, draw a diagram showing what orbitals are used to form each bond.
(c) There are two different substances with the formula C4H10. Draw both, and tell how they differ.
|
Create a good project based on online diary
: Create a good project based on online diary and event management.users can register and create daily/weekly/yearly events,create contacts,upload media and sent messages to their friends.
|
|
Prepare a trial balance as of february
: Prepare a trial balance as of February 28, 2014. Business Applicatio- Examine the transactions for February 3, 9, 10, and 22.
|
|
Passenger fell beneath the wheels of the train and exploded
: 1)A passenger ran after a train as it was leaving a station. Two railroad employees boosted the passenger aboard, but as they did so a package carried by the passenger fell beneath the wheels of the train and exploded. The package
|
|
What is the maximum volume box could have
: A box is to be constructed from a sheet of cardboard that is 20 cm by 60 cm, by cutting out squares of length x by x from each corner and bending up the sides. What is the maximum volume this box could have?
|
|
Calculate the partial pressures of helium
: Calculate the partial pressures of helium and neon after the stopcock of the following apparatus is open. Assume that the temperature remains constant at 16°C.
|
|
How the store can increase profitability
: Just a page to page and half writing needs to be done. For sports check the store. Make 1-2 recommendation for how the store can increase profitability using consumer behavior concepts. Keeping in mind this is a Marketing course on Consumer Beahviour..
|
|
What does this gap measure indicate
: Calculate the six month GAP associated with this transaction. What does this GAP measure indicate about interest rate risk in this transaction? Calculate the three month GAP associated with this transaction. Is this a better GAP measure of the bank's..
|
|
Describe the difference between elements and compounds
: Explain why Brownian motion provides evidence for the existence of atoms and molecules. Describe the Difference between elements, compounds, and mixtures. Which would be easier to separate, a mixture or a compound? Explain your answer
|
|
Mergers and acquisitions
: Write at least one paragraph for each concept under each number. Clearly identify the concept in each paragraph including an explanation or definition of what it means. In addition, explain how and why it is important to you, including how you thin..
|