Reference no: EM132043
Question 1:
Heat capacities Cv and Cp are defined as temperature derivatives respectively of U and H. Show that the general expression connecting Cp to Cv is:
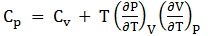
Question 2
Liquid isobutane is throttled through a valvefrom an initial state of 360 K and 4000 kPa to a final pressure of 2000 kPa. Estimate the entropy change of the isobutane. The specific heat of liquid isobutane at 360 K is 2.78 J/g/°C.
For volume calculation, use equation:
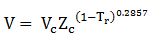
Given: Vc= 262.7 cm3/mol, Zc= 0.282 and Tc= 408.1 K
Also, change in T and V is negligible during throttling process.
Question 3
A concentrated binary solution containing mostly species 2 (but x2 not equals to 1) is in equilibrium with a vapor phase containing both species 1 and 2. The pressure of this two-phase system is 1 bar; and the temperature is 25°C. Determine from the following data good estimates of x1 and y1.
Given: H1 = 200 bar, P2sat = 0.1 bar
State and justify all assumptions.
Question 4
For the acetone(1)/methanol(2) system a vapor mixture for which z1 = 0.25 and z2 = 0.75 is cooled to temperature T in the two-phase region and flows into a separation chamber at a pressure of 100 kPa. If the composition of the liquid product is to be x1= 0.175, what is the required T and what is the value of y1. Also calculate the molar fraction of the system in the vapor phase.
Given: lnγ1 = 0.64 x22
lnγ2 = 0.64 x12
ln(Psat) = A - B/C + T(oc)
where, for Acetone: A = 14.3916 ; B = 2795.82 ;C = 230.00
for Methanol: A = 16.5938 ; B = 3644.30 ; C = 239.76