Reference no: EM131488696
Question:
Show that the emission of hydrogen atom from n=6 to n=2 does have a wavelength of 410 nm.
Given that the energy of hydrogen-like atom is written as: (all of them are equivalent)
Part 1 - Familiarize with equations
Write down the equation of: Example: Energy Single Electron Atom
En = -13.6eV (z2/n2)
1. Energy Multi Electron Atom.
2. Energy Particle in a Box.
3. Photoelectric Effect (wavelength, work function, and speed of e-).
4. Energy of Photon based on frequency.
Part 2 - True or False
In a Single electron atom, energy of orbitals: 3s = 3p = 3d.
In a Multi electron atom, energy of orbitals: 3s = 3p = 3d.
In a Single electron atom, the effective nuclear charge experienced by an electron is always equal to the number of proton.
In a Multi electron atom, the effective nuclear charge experienced by an electron is always equal to the number of proton.
Zeff is the effective nuclear charge experienced by an electron.
Based on the equation of Multi electron atom, the higher the Zeff, the lower the energy (more negative).
In a Single electron atom: Z effective of 3s = 3p = 3d.
In a Multi electron atom: Z effective of 3s > 3p > 3d.
λDB = h/mv This equation express the wave-length of a particle.
λ = c/v This equation express the wave-length of light.
Part 3 - Guided question
1) There is a convenient conversion that you can use in this class:
Energy(in eV) = (1240 nm.eV/λ(in nm))
This equation is equivalent to E = hc/λ
i) Calculate the λ of a photon with an energy of 5eV.
ii) Calculate the Energy of a photon with λ = 300 nm. Express answer in eV.
iii) Convert 20 eV to Joule.
2) Consider the quantum system below:
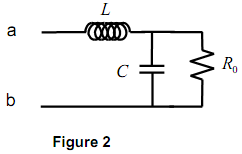
i) Calculate the energy of photon (in eV) emitted by an electron transitioning from C to B. SHOW APPROACH.
ii) Calculate the wavelength from of photon emitted by an electron transitioning from C to B.
iii) Calculate the wavelength of a photon emitted by an electron transitioning from C to A.
3) An electron in a Particle in a Box model initially at n = 4 absorb a photon to n = 7. The length of the box "L" is 4x10-9m.
i) Calculate the energy of n=7. Express answer in Joule
ii) Calculate the energy of n=4. Express answer in Joule.
iii) Calculate the energy difference n=7 and n=4.
iv) Convert energy from Joule to eV.
v) Calculate the wavelength of photon necessary to excite the electron of PIB from n=4 to n=7.
4) For an Iron molecule (Fe), the effective nuclear charge are as follow:
Zeff of 4s = 3.75 and Zeff of 3d = 6.75
i) Calculate the energy of 4s electron (in eV).
ii) Calculate the energy of 3d electron (in eV).
iii) Calculate the energy of electron in Iron if n=∞.
iv) Calculate the energy difference of 4s and 3d orbital.
v) Calculate the wavelength of photon necessary to excite an electron in 4s orbital to 3d orbital.
vi) Ionization energy is a transition from an initial quantum state to n=∞. Calculate ionization energy of 4s and 3d electron in iron.
5) Drawing