Reference no: EM13840120
EXPERIMENTS IN GENERAL CHEMISTRY
Additional Pre-Lab Components
Experiment 1:
Measured properties are characterized by their accuracy and their precision. When using measured values in calculations, it is most important to note that precision can neither be gained nor lost. The rules for significant figures usually obey this principle, but sometimes the result has to be adjusted to maintain precision. Use the following example to illustrate these ideas:
1. A liquid was measured to occupy a volume of 4.8 mL using a graduated cylinder, and its mass was determined using balances of different precision:
Triple-beam balance (precision of 1 dec. pl.): m = 5.3 g Analytical balance (precision of 3 dec. pl.): m = 5.261 g Show that both mass-measurements, combined with the volume measurement, yield the same value for the density of the liquid.
2. A high-density liquid (d = 2.32 g/cm3) was measured to occupy a volume of 49.4 mL using a graduated cylinder. Report the mass of the liquid with correct precision. Is this result in accordance with the rules for significant figures?
When measuring volumes, the correct reading of the meniscus is key to producing reliable results. Use the following example to illustrate this idea:
3. A liquid was measured to occupy a certain volume using a graduated cylinder, and its mass was determined using balances of different precision. Two experimenters report two different values for the density:
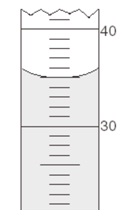
Experimenter 1:
m = 37.598 g (Analytical balance)
d = 1.0 g/mL
Experimenter 2:
m = 37.6 g (Triple-beam balance)
d = 1.1 g/mL
Decide which experimenter reports a correct result, and explain where the other experimenter went wrong.
Experiment 2:
Chemical equations serve a variety of purposes, but the most general form of a chemical equation is reactants àà products. Most often, reactants and products are described by their chemical formula, possibly including a designation of state of matter. Contrary to a general chemical equation, a balanced chemical equation always uses molecular formulas and is amended by stoichiometric factors to assure conservation of mass and/or moles.
Chemical equations represent chemical reactions, and chemical reactions can be classified according to two groups:
Group I (bond breakage and/or bond formation): The four reaction types are Synthesis, Decomposition, Single Displacement, Double Displacement.
Group II (common reaction principles): Representative examples are Precipitation, Redox, Acid-Base, Combustion.
Choose from the following general chemical equations, and illustrate the four principle group I reaction types, and the four representative group II reaction types:
A) aqueous potassium sulfate + aqueous barium nitrate àà aqueous potassium nitrate + solid barium sulfate
B) magnesium metal + nitrogen gas àà solid magnesium nitride
C) copper metal + aqueous silver nitrate àà aqueous copper nitrate + silver metal
D) chlorine gas + fluorine gas àà chlorine monofluoride gas
E) aqueous hydrochloric acid + aqueous calcium hydroxide àà aqueous calcium chloride + water
F) gaseous sulfur dioxide + oxygen gas àà gaseous sulfur trioxide
G) solid calcium carbonate àà solid calcium oxide + gaseous carbon dioxide
H) solid silver oxide àà oxygen gas + silver metal
When you discuss reaction types, formulate the chosen general equation as balanced chemical equation.
Any given equation might serve as an example for a group I as well as for a group II reaction, but try to use as many different equations as possible.
Experiment 3:
When chemical reactions are carried out under well-defined conditions, mass differences provide an access to chemical compositions and empirical formulas. However, when no steps are included that correct for certain aspects of the reaction, or when the reaction conditions are not carefully controlled, one might obtain erroneous chemical formulas. One example is the synthesis of metal oxides. When the combustion of a metal is carried out in an oxygen atmosphere, the only products obtained are metal oxides. However, when carried out in air, part of the metal forms metal nitrides as well. Another example is the determination of crystal water. If carefully executed, the anhydrous compound is formed, and the hydration number is most often integer. If not heated sufficiently, not all crystal water is driven out. If overheated, the product might undergo thermal decomposition. Use the following examples to illustrate these ideas.
1. When a 1.50 g sample of aluminum metal is burned in an oxygen atmosphere, 2.83 g of aluminum oxide are produced. However, the combustion of 1.50 g ultrafine aluminum in air results in 2.70 g of a product, which is a mixture of 80% aluminum oxide and 20 % aluminum nitride (% by mass). Use this information to determine the empirical formulas of aluminum oxide and aluminum nitride.
2. Samples of hydrated manganese perchlorate are heated to determine the amount of crystal water:
Mn(ClO4)2·nH2O àà Mn(ClO4)2 + nH2O Two samples of different weight are studied:
a) m (before heating) = 1.629 g ; m (after heating) = 1.142 g
b) m (before heating) = 9.048 g ; m (after heating) = 6.645 g
For both samples, calculate the number of moles of crystal water per formula unit of manganese perchlorate. Decide which sample is most likely to represent the correct result, and explain what might have gone wrong with the other.
Experiment 4:
When sending copper metal through a cycle of reactions, it is most important to have an idea how much of any given reagent is needed to bring each step of the cycle of reactions to completion. In order to assure a complete reaction, reagents are often added in excess (up to 10 fold), but too much as well as too little might hamper the course of a reaction. The five key steps of the copper cycle are shown below:
Step 1: 3 Cu(s) + 2NO3- + 8H+ àà 3 Cu2+(aq) + 4 H2O + 2 NO
Step 2: Cu2+(aq) + 2 OH-(aq) àà Cu(OH)2(s)
Step 3: Cu(OH)2(s) àà CuO(s)+ H2O
Step 4: CuO(s) + 2 H+ àà Cu2+(aq) + H2O
Step 5: Cu2+(aq) + Zn(s) àà Zn2+(aq) + Cu(s)
Assume that you want to carry out a sequence of cycle reactions based on 1.00 g of copper. In order to use the right amount of reagents, present answers to the following questions:
1. What volume of 16 M HNO3 is required to completely react with Cu in the first step of the cycle?
2. What volume of 3.0 M NaOH is required to precipitate all copper(II) cations as Cu(OH)2 in the second step of the cycle?
3. How many grams of copper(II)oxide will form when the third step of the cycle goes to completion?
4. What volume of 6.0 M H2SO4 is required to completely convert all copper(II)oxide to copper(II) cation in the fourth step of the cycle?
5. How many grams of zinc metal are needed to completely regenerate all copper in the fifth step in the cycle?
Experiment 5:
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Although advanced quantitative pH- considerations often result in fairly acidic calculations, even a basic qualitative pH-assessment requires a good understanding of elemental concepts and ideas. Thus, you should be able to provide a meaningful answer to the following essential questions:
1. How is pH related to the concentration of hydrogen cations [H+]?
2. What is understood by the auto-ionization of water?
3. What is the ion product of water?
4. What is the pH of a neutral aqueous solution (neither acidic nor basic)?
5. How is the pH of a neutral aqueous solution related to the ion product of water?
6. How is pH related to the concentration of hydroxide anions [OH-]?
7. How many significant figures are contained in the following value: pOH = 10.45?
8. What is a pH indicator?
Experiment 6:
The specific heat of nickel is measured using a simple experimental setup. Besides a sample of nickel, all that is needed for this experiment are hot as well as cold water, Styrofoam cups and a thermometer.
1. The Styrofoam cups function as calorimeter, and before the heat capacity of nickel is measured, the heat capacity of the calorimeter needs to be determined. Hot water and cold water are mixed in the Styrofoam cups, and the change in temperature is measured. From the following data, calculate the heat capacity Ccal of the calorimeter:
Mass of empty Styrofoam cups: 6.2 g
Mass of cups + 70 mL H2O 74.6 g Mass of cups + 70 mL H2O + 30 mL hot H2O 99.1 g Initial temperature of water in cups 24.0 °C
Temperature of boiling water bath: 99.5 °C
Final temperature of water: 40.1 °C
2. Having established a value for Ccal, the same calorimeter is used to determine the specific heat of nickel. To do so, a 78.8 g sample of nickel is heated to 99.2 °C and transferred to 99.2 mL of water at 26.1 °C. The final temperature of the water and metal is found to be 32.1 °C. What is the specific heat sNi of nickel?
3. Finally, the accuracy of the thermometer is checked measuring the boiling point and the freezing point of water. The thermometer reads 102.0 °C when water is boiling and 2.0 °C at the freezing point of water. Although the thermometer is clearly not calibrated, explain why this will not affect the results of the experiment.
Experiment 7:
When performing acid-base titrations, the first step is often a standardization of the titrant, which is an accurate determination of its concentration. Acidic titrants might be standardized in titration of a well-defined amount of a solid base, such as sodium carbonate. In such a procedure, why is it not necessary to know the exact amount of water used in preparation of a solution of Na2CO3 used in standardization of an acid?
The standardization of an acid might also be carried out using a solution of a base with known concentration. For example, it is found that 37.60 ml of 0.210 M NaOH are required to neutralize
25.05 ml of H2SO4 solution in a titration experiment. Calculate the molarity and normality of the H2SO4 solution.
In the example given above, the amount of acid to be titrated has to be delivered by a pipet so that its volume is accurately known. However, the solution of the acid is often diluted to facilitate the titration process. Explain why additional de-ionized water might be added without affecting the results of the titration.
Experiment 8:
The Dumas Method is a simple procedure to determine the molar mass of volatile liquid. In such an experiment, a precisely known amount of the volatile liquid is transferred from the liquid state to the gas phase, and the ideal gas law in combination with the mass of the gas provides an easy access to the molecular weight of the volatile liquid.
To illustrate this, consider the determination of the molar mass of diethyl ether. The following set of data was collected in a Dumas experiment:
Atmospheric pressure: 756.2 mm Hg Temperature of the boiling water: 99.6 °C
Mass of the empty flask: 135.263 g Mass of flask + condensed liquid: 135.886 g Volume of water required to fill flask: 262.2 mL
Calculate the molar mass of diethyl ether from this data.
The success of this simple method depends on a lot of things going right, since it is based on various assumptions. Explain which assumptions are made when applying the Dumas Method to determine the molecular weight of a volatile liquid.
The molecular weight then allows one to determine the density of the gas vapor. Use data of the example given above, and calculate the density of gaseous diethyl ether at atmospheric pressure and the temperature of boiling water.
Experiment 9:
This experiment explores Hess' Law, a cornerstone of thermochemistry. The enthalpy of reaction ?H for two acid-base reactions serves as key property to employ and confirm Hess' law.
The two acid-base reactions utilize a strong acid - HCl - as well as a weak acid - CH3COOH. The base used is solid sodium hydroxide.
1. The heat of neutralization of solid sodium hydroxide with an aqueous solution of an acid might be determined directly in a one-step reaction or indirectly using a two-step reaction. Explain the difference between a one-step and a two-step reaction mechanism when determining the heat of neutralization.
2. Would you expect different values for the heat of the neutralization reaction, when solid NaOH is neutralized with an aqueous solution of a weak acid or with an aqueous solution of a strong acid? Explain your answer.
3. Explain how the fact that the weak acid is only partially dissociated might cause discrepancies between a theoretical predicted and an experimentally determined value.