Zinc:
(1) Occurrence of zinc: Since zinc is a reactive metal so it does not occur in the native form. The chief ores of zinc are
(a) Zinc blende (ZnS)
(b) Calamine or zinc spar (ZnCO3) and
(c) Zincite (ZnO)
(2) Extraction of zinc: Zinc blende, while the concentration through Froth floatation method zinc is roasted in air to convert it into ZnO. With the case of calamine, ore is calcined to get ZnO. The oxide thus acquired is heated with the temperature of 1673 K and mixed with the crushed coke and in fire clay retorts (Belgian Process) while ZnO gets decreased to metallic zinc. It being volatile at this temperature, a metal distils over and is condensed leaving behind Cd or Pb, Fe as impurities. Crude metal is also known as spelter metal. The metal could be refined either through the electrolysis or else by fractional distillation.
Properties of Zn:
As compare to the mercury Zinc is more reactive. This is a fine conductor of electricity and heat. Zinc simply merges along with the oxygen to form ZnO. A pure zinc do not react with the non-oxidising acids (HCl or H2SO4) but impure metal reacts forming Zn2+ ions and evolving H2 gas.
Hot and conc. H2SO4 attacks zinc liberating SO2 gas in the given equation
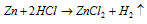
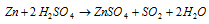
Zinc also reacts with both conditions (hot and cold) conc and HNO3. HNO3 liberating nitrous oxide (N2O), ammonium nitrate (NH4NO3) and nitrogen dioxide (NO2) correspondingly.
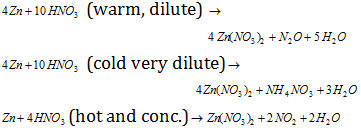
Zinc dissolves in hot concentrated NaOH creating the soluble sod. Zincate the reaction of zincate is given below:
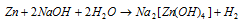
Or
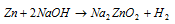
(3) Special varieties of zinc.
These both varieties of zinc are used as the reducing agents within laboratory.
(i) Granulated zinc: It could be prepared through pouring molten zinc within the cold water.
(ii) Zinc dust: It is prepared through melting zinc and then atomizing it with a blast of air.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Zinc questions? Zinc topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Zinc related problems. We provide step by step Zinc question's answers with 100% plagiarism free content. We prepare quality content and notes for Zinc topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours