Bonding in co-ordination compounds (Werner's Coordination theory)
The scientist named Werner was able to explain the bonding in complex.
Primary valency (Pv) : This is non- directional and ionizable. Actually it is the positive charge on the metal ion.
Secondary valency (Sv) : This is directional and non- ionizable. It is equal to number of the ligand atoms co-ordinated to the metal (co-ordination number). Example of it can be seen in the table which is drawn below :
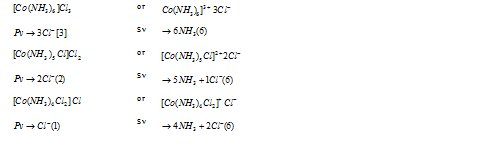
The nature of the complex may be understood by treating the above complexes with excess of AgNo3
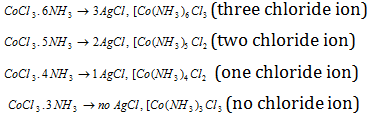
The nature of bonding between central metal atom and ligands in the coordination sphere has been explained by the three well-known theories.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Werner's Coordination theory questions? Werner's Coordination theory topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Werner's Coordination theory related problems. We provide step by step Werner's Coordination theory question's answers with 100% plagiarism free content. We prepare quality content and notes for Werner's Coordination theory topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours