Water
Water is the oxide of hydrogen. It is an essential component of animal and vegetable matter. Water constitutes about 65% of our body. It is the principal constituent of earth's surface.
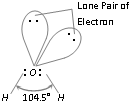
(1) Structure : Due to the presence of lone pairs, geometry of water is the distorted and the H-O-H bond angle is 104.5°, which is much less than the normal tetrahedral angle (109.5°). The geometry of the molecule is regarded as angular or bent. In water, each O-H bond is polar because of the high electronegativity of oxygen (3.5) in comparison to that of hydrogen (2.1). The resultant dipole moment of water molecule is 1.84D.
In ice, each oxygen atom is tetrahedrally surrounded by four hydrogen atoms; two by covalent bonds and two by hydrogen bonds. The obtained structure of ice is open structure having a number of vacant spaces. Thus, the density of ice is less than that of water and ice floats over water. It can be noted that water posses maximum density (1 gm cm-3) at 4°C (277 K).
(2) Heavy water: Chemically heavy water is deuterium oxide (D2O). It was discovered by Urey.
It is attained as the byproduct in some of the industries where is produced by the electrolysis of water.
Heavy water (D2O) is used
(a) as the moderator and coolant in the nuclear reactors
(b) in study of the mechanism of chemical reactions
(c) as a starting material for the preparation of a number of the deuterium compounds, which are given as follows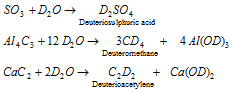
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Water questions? Water topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Water related problems. We provide step by step Water question's answers with 100% plagiarism free content. We prepare quality content and notes for Water topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours