Volumetric analysis: This is a method which involves the quantitative determination of the amount of any substance present in the solution through volume measurements. For analysis the standard solution is needed. (A solution which contains a known weight of solute present in known volume of solution is known as standard solution.)
To determine strength of the unknown solution with the help of known (standard) solution is generally known as titration. Different types of titrations are possible which are summarized as follows,
(i) Redox titrations: To find out the strength of an oxidising agents or reducing agents by titration with the help of standard solution of the reducing agents or oxidising agents.
Examples:
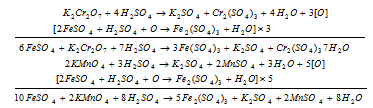
Similarly with H2C2O4
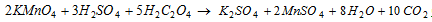
(ii) Acid-base titrations : To find out the strength of acid or base with the help of standard solution of base or acid.
Example: 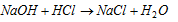
and 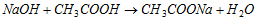 etc.
etc.
(iii) Iodiometric titrations : This is a simple titration involving free iodine. This includes the titration of iodine solution with known sodium thiosulphate solution whose normality is N. Let the volume of sodium thiosulphate is V ml.
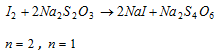
Equivalents of I2 = Equivalent of Na2S2O3
Equivalents of I2 = N * V * 10-3
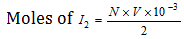
Mass of free I2 in the solution 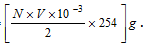 .
.
(iv) Iodometric titrations : This is the indirect method for the estimation of the iodine. An oxidising agent is made to react with the excess of solid KI. The oxidising agent oxidises I- to I2. This iodine is then made to react with the Na2S2O3 solution.
Oxidising Agent
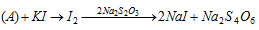
Let the normality of Na2S2O3 solution is N and the volume of thiosulphate consumed to V ml.
Equivalent of A = Equivalent of I2 = Equivalents of Na2S2O3
Equivalents of I2 liberated from KI = N * V * 10-3
Moles of I2 liberated from 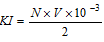
Mass of liberated from 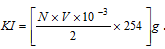 .
.
(v) Precipitation titrations : To determine the anions like 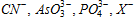 etc, by precipitating with AgNo3 provides examples of precipitation titrations.
etc, by precipitating with AgNo3 provides examples of precipitation titrations.
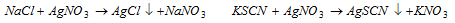
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Volumetric analysis questions? Volumetric analysis topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Volumetric analysis related problems. We provide step by step Volumetric analysis question's answers with 100% plagiarism free content. We prepare quality content and notes for Volumetric analysis topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours