Vander Waal's equation
(1) To rectify the errors caused by ignoring the intermolecular forces of attraction and the volume occupied by molecules, Vander Waal (in 1873) modified the ideal gas equation by introducing two corrections,
(i) Volume correction (ii) Pressure correction
(2) Vander Waal's equation is obeyed by the real gases over wide range of temperatures and pressures, hence it is called equation of state for the real gases.
(3) The Vander Waal's equation for n moles of the gas is,
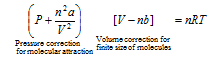
a and b are Vander Waal's constants whose values depend on the nature of gas. Generally for a gas a>>b.
(i) Constant a : It is a indirect measure of magnitude of attractive forces between the molecules. Greater is the value of a, more easily the gas can be liquefied. Therefore the easily liquefiable gases (such as SO2>NH3> H2S> CO2) have high values than the permanent gases (such as N2>O2>H2>He).
Units of 'a' are: atm.L2 mol-2 or atm.m6 mol-2 or N m4 mol-2 (S.I. unit).
(ii) Constant b : Also called co-volume or excluded volume,
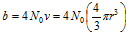
It's value gives an idea about the effective size of the gas molecules. Greater is value of b, larger will be the size and smaller is the compressible volume. As b is the effective volume of gas molecules, constant value of b for any of the gas over the wide range of temperature and pressure indicates that the gas molecules are incompressible.
Units of 'b' are : L mol-1 or m3mol-1 (S.I. unit)
(iii) The two Vander Waal's constants and Boyle's temperature (TB) are related as,
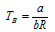
(4) Vander Waal's equation at different temperature and pressures
(i) When pressure is extremely low : For one mole of the gas,
or 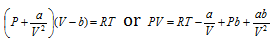
(ii) When pressure is extremely high : For one mole of the gas,
; or 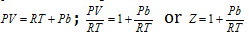
where Z is compressibility factor.
(iii) When temperature is extremely high : For one mole of the gas,
PV =RT.
(iv) When pressure is low : For one mole of the gas,
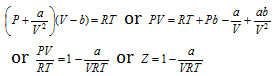
(v) For hydrogen : Molecular mass of hydrogen is small hence value of 'a' will be small owing to smaller intermolecular force. Thus the terms 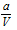 and
and 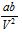 may be ignored. Then Vander Waal's equation becomes,
may be ignored. Then Vander Waal's equation becomes,
or 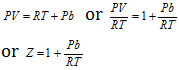
In case of the hydrogen gas, compressibility factor is always greater than one.
(5) The merits of Vander Waal's equation
(i) The Vander Waal's equation holds good for real gases upto moderately high pressures.
(ii) The equation represents the trend of the isotherms representing the variation of PV with P for various gases.
(iii) From the Vander Waal's equation it is possible to obtain expressions of Boyle's temperature, critical constants and inversion temperature in terms of the Vander Waal's constants 'a' and 'b'.
(iv) The Vander Waal's equation is quite useful in obtaining a reduced equation of state which being a general equation of state has the advantage that a single curve can be obtained for all gases when the equation if graphically represented by plotting the variables.
(6) Limitations of Vander Waal's equation
(i) This equation shows appreciable deviations at too low temperatures or too high pressures.
(ii) The values of the Vander Waal's constants a and b do not remain constant over the entire ranges of T and P, thus this equation is valid only over specific range of T and P.
(7) Other equations of state : In addition to Vander Waal's equation, there are equations of state also which have been used to explain real behavior of gases are,
(i) Clausius equation : 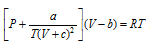 . Here c is another constant in addition to b, a and R.
. Here c is another constant in addition to b, a and R.
(ii) Berthelot equation : 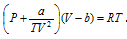 .
.
(iii) Wohl equation : 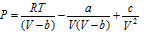
(iv) Dieterici equation : 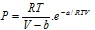 .
.
This expression is obtained on the basis of the concept that molecules near the wall will have higher potential energy than those in the bulk.
(v) Kammerlingh Onnes equation : It is the most general or satisfactory expression as equation expresses PV as a power series of P at a given temperature.
PV = A+BP+CP2+DP3+........
Here coefficients A, B, C etc. are known as first, second and third and more virial coefficients.
(a) The virial coefficients are different for the different gases.
(b) At extremely low pressure, first virial coefficient, A = RT.
(c) At high pressure, other virial coefficients also become important and must be considered.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Vander Waal's equation questions? Vander Waal's equation topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Vander Waal's equation related problems. We provide step by step Vander Waal's equation question's answers with 100% plagiarism free content. We prepare quality content and notes for Vander Waal's equation topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours