The Valence Bond theory of coordination compounds
(i) The suitable number of the atomic orbitals of central metal ion (s, p, d) hybridise to give empty hybrid orbitals.
(ii) These specific hybrid orbitals accept lone pair of the electrons from ligands and are directed towards the ligand positions according to the geometry of complex.
(iii) When inner d-orbitals that is (n-1) d orbitals are used in the process of hybridization, the complex is called as inner orbital or spin or the hyperligated complex.
(iv) A substance which do not contain any unpaired electron is not attracted by 2 magnet. It is said to be diamagnetic. Conversely, a substance which contains one or more unpaired electrons in electrons in the d-orbitals, is attracted by the magnetic field [exception O2 and NO]. It is said to be paramagnetic.
The paramagnetism can be calculated by expression stated below,
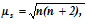
here μ magnetic moment.
s= spin only value and n= number of unpaired electrons.
Therefore, if 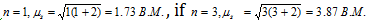 and so on
and so on
On the basis of value of magnetic moment, we can predict the number of the unpaired electrons present in complex. If we are aware of the number of unpaired electrons in the metal complex, then it can be possible to predict the geometry of complex species.
(v) There are two types of ligands mainly strong field and weak field ligands. The strong field ligand is able to force the electrons of the metal atom/ion to pair up (if required). The pairing of them cannot be done only to the extent which is needed to cause the hybridization possible for that co-ordination number. The weak field ligand is incapable of making electrons of the metal atom/ ion to pair up.
Strong field ligands : CN-, CO, en, NH3, H2O, No-, Py.
Weak field ligands :
I-. Br-, Cl-, F-, NO3-, OH-, C2O42-, NH3, H2O.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Valence Bond theory of Co-ordination Compounds questions? Valence Bond theory of Co-ordination Compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Valence Bond theory of Co-ordination Compounds related problems. We provide step by step Valence Bond theory of Co-ordination Compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for Valence Bond theory of Co-ordination Compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours