Types of heat of reaction
(i) Heat of formation : It is the quantity of heat evolved or absorbed (i.e. the change in enthalpy) when one mole of the substance is formed from its constituent elements under given conditions of temperature and pressure. It is represented by ΔHf. When the temperature and pressure are as 25°C and 1 atmospheric pressure. The heat of formation under these conditions is termed as standard heat of formation. It is usually represented by ΔH0f .
Standard heat of the formation of 1 mole of NH3(g) and 1 mole of HCl(g).
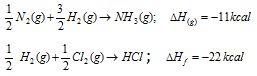
It may be calculated by
(ii) Heat of combustion: It is the amount of heat evolved or absorbed (i.e. change in enthalpy) when one mole of the substance is completely burnt in air or oxygen. The examples are given as follows
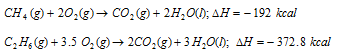
It may be calculated by

The enthalpy or heat of combustion has a number of applications. Some applications of it are described below,
(a) Calorific value of foods and fuels: Energy is needed for the working of all machines. Even the human body is no exception. Petroleum, natural gas coal, etc acts as the principal sources of energy for man-made machines, food which we eat acts as a source of energy to our body.
The energy released by the combustion of foods or fuels is usually compared in terms of their combustion energies per gram. It is called as calorific value. The quantity of heat produced in calories or Joules when one gram of a substance (food or fuel) is completely burnt or oxidised.
When methane burns, 890.3 kJ mol-1 of energy is released.
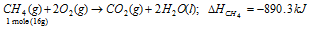
So, the calorific value of methane = 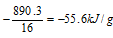
(b) The Enthalpies of formation: Enthalpies of formation of the various compounds, which are not directly obtained, can be calculated from the data of enthalpies of combustions easily by the application of Hess's law.
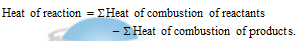
(iii) Heat of neutralization: It is the amount of heat evolved (i.e., change in enthalpy) when one equivalent of an acid is neutralized by one equivalent of a base in fairly dilute solution, for example Neutralization reactions are always exothermic reaction and the value of ?H is (-ve).
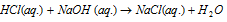
?H=-13.5kcal
The heat of neutralization of a strong acid against a strong base is always constant 13.7kcalor57kJmole-1. It is because in dilute solutions all strong acids and bases ionize completely and thus the heat of neutralization in such cases is actually the heat of formation of water from H+ and OH- ions, i.e.,
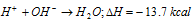
In case of neutralisation of a weak acid or a weak base against a strong base or acid respectively, since a part of the evolved heat is used up in ionising the weak acid or base, it is always less than 13.7 Kcal mole-1 (57 KJ mole-1). For example,
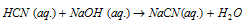
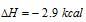
HCN(aq)? H+ + CN-; DH = 10.8 Kcal
10.8Kcal of heat is absorbed for ionization of HCN it is heat of dissociation or ionization
(iv) Heat of solution: It is the amount of heat evolved or absorbed (i.e., change in enthalpy) when one mole of the solute is dissolved completely in excess of the solvent (commonly water). For example,
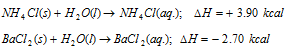
(v) Heat of hydration: It is the amount of heat evolved or absorbed (i.e change in enthalpy) when mole of an anhydrous or a partially hydrated salt combines with the required number of moles of water to form a specific hydrate. For instance,
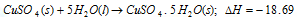
(vi) Heat of vaporization: When a liquid is allowed to evaporate, it absorbs heat from surroundings and the evaporation is accompanied by increase in enthalpy. For example: 10.5kcals is the increase in enthalpy when one mole of water is allowed to evaporate at 25°C. When the vapours are allowed to condense to liquid state, heat is given out and condensation of the vapour is accompanied by the decrease in enthalpy.
The representation of evaporation and condensation can be done as follows,
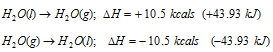
Hence the change in enthalpy when a liquid changes into the vapour state or when vapour changes into liquid state is called heat of vapourisation.
(vii) Heat of fusion : When a solid is allowed to melt, it changes into the liquid state with the absorption of heat (increase in enthalpy) and when a liquid is permitted to freeze, it changes into solid with the evolution of heat (decrease in the enthalpy). The transform in enthalpy of such type of transformations is termed as enthalpy of the fusion. The example is as follows
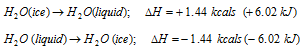
(viii) Heat of precipitation : It is defined as the amount of heat liberated in the precipitation of one mole of a sparingly soluble substance when solutions of suitable electrolytes are mixed, for example
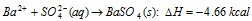
(ix) Heat of sublimation : The Sublimation is the process in which a solid on heating changes directly into the gaseous state below its melting point.
The Heat of sublimation of the substance is amount of heat absorbed in the conversion of 1 mole of a solid directly into vapour phase at a given temperature below its melting point.

Almost all the solids that sublime are molecular in nature such as iodine and naphthalene etc.
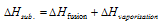
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Types of heat of reaction questions? Types of heat of reaction topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Types of heat of reaction related problems. We provide step by step Types of heat of reaction question's answers with 100% plagiarism free content. We prepare quality content and notes for Types of heat of reaction topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours