Types of catalysis
The catalytic reactions can be broadly divided into following types,
(1) Homogeneous catalysis : When the reactants and the catalyst are in the same phase (i.e. solid, liquid or gas). The catalysis is said to be homogeneous. The below given are some of the examples of homogeneous catalysis.
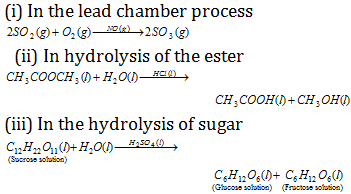
(2) Heterogeneous catalysis : The catalytic process in which the reactants and the catalyst are in different phases is known as heterogeneous catalysis. Some of the examples of heterogeneous catalysis are given below.

(3) Positive catalysis : When the rate of the reaction is accelerated by the foreign substance, it is said to be a positive catalyst and phenomenon as positive catalysis. Some examples of positive catalysis are given below.
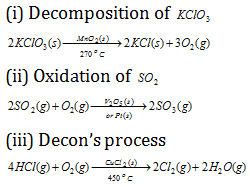
(4) Negative catalysis : There are some, substance which, when added to the reaction mixture, delay the reaction rate instead of increasing it. These are termed as negative catalyst or inhibitors and the phenomenon is known as negative catalysis. Some instances of it are as follows.
(i) Oxidation of sodium sulphite
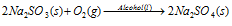
(ii) Oxidation of benzaldehyde
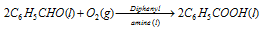
(iii) Tetra ethyl lead (TEL) is added to petrol to retard the ignition of petrol vapours on compression in an internal combustion engine and thus minimise the knocking effect.
(5) Auto-catalysis: In some reactions, one of the product acts as a catalyst. In the early stages the reaction is slow but as soon as the products come into existences the reaction rate increases. This type of process is called as auto-catalysis. Some examples it are as follows,
(i) The rate of oxidation of oxalic acid by acidified potassium permanganate increases as the reaction progresses. This acceleration is because of the presence of Mn2+ ions which are formed during reaction. Therefore Mn2+ ions act as auto-catalyst.
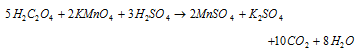
(ii) At the time when nitric acid is poured on the copper, the reaction is very slow in the beginning; gradually reaction becomes faster because of the formation of nitrous acid during the reaction which acts as an auto-catalyst.
(6) Induced catalysis: When one reaction influences the rate of other reaction, which does not take place under ordinary conditions, the process is called as induced catalysis. Some examples of it are as follows,
(i) Sodium arsenite solution is not oxidised by air. If, still, air is passed through a mixture of the solution of sodium arsenite and sodium sulphite, both of them undergo the simultaneous oxidation. The oxidation of sodium sulphite, hence, induces the oxidation of sodium arsenite.
(ii) The reduction of mercuric chloride (HgCl2) with oxalic acid is quite slow, but the potassium permanganate is reduced readily with the oxalic acid. If, however, the oxalic acid is added to a mixture of potassium permanganate and HgCl2 both are reduced at the same time. The reduction of potassium permanganate, hence, induces the reduction of mercuric chloride.
(7) Acid-base catalysis: According to the Arrhenius and Ostwald H+ or H- ion act as a catalyst.
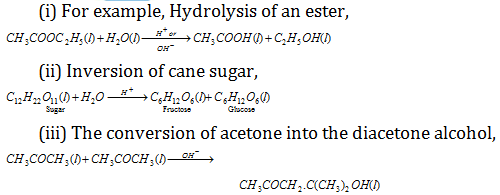
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Types of Catalysis questions? Types of Catalysis topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Types of Catalysis related problems. We provide step by step Types of Catalysis question's answers with 100% plagiarism free content. We prepare quality content and notes for Types of Catalysis topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours