The two theories have been proposed to explain change of colour of acid-base indicators with the change in pH.
(i) Ostwald's Theory (ii) Quinonoid theory
(1) Selection of suitable indicator or choice of indicator : In order to choose a suitable indicator, it is important to understand the pH changes in the titrations. The change in the pH value in the vicinity of the equivalence point is most important for this purpose. The curve attained by plotting pH value as ordinate against the volume of alkali added as abscissa is known as neutralisation or titration curve. The appropriate indicators for the following titrations are,
(i) Strong acid Vs strong base : Phenolphthalein (pH range from 8.3 to 10.5), methyl red (pH range from 4.4 - 6.5) and methyl orange (pH range from 3.2 to 4.5).
(ii) The weak acid versus strong base : Phenolphthalein.
(iii) Strong acid versus weak base : Methyl red and methyl orange.
(iv) Weak acid versus weak base : No suitable indicator can be used for such a titration.
Reason for use of different indicators for different systems : Indicators are either weak acids or weak bases and when dissolved in water their dissociated form acquires a colour different from that of the undissociated form. Take a weak acid indicator of the general formula HIn, wherein represents indicator. The equilibrium in aqueous solution will be
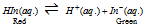
Let be the equilibrium constant
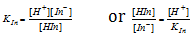
The human eye can detect change in colour if the ratio of two forms of indicator ranges from 0.1 to 10.
If, 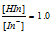 , the colour visible is yellow
, the colour visible is yellow
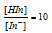 , the colour visible is red.
, the colour visible is red.
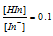 , the colour visible is green.
, the colour visible is green.
Or we can say that
The colour visible to us will be red, when the 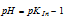
The colour visible to us will be yellow, when the 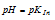
The colour visible to us will be green, when the 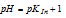
Therefore, our imaginary indicator will be red at any pH which just falls below 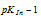 and green at any pH which just exceeds
and green at any pH which just exceeds 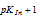 . The indicator changes its colour in the narrow pH range
. The indicator changes its colour in the narrow pH range 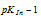 to
to 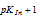 from red to (yellow, yellow-green, red-yellow) green. We can hence use this indicator to locate this narrow pH range. In other words, in order to use the indicator effectively in this range, we should have a solution for which pH is very near to
from red to (yellow, yellow-green, red-yellow) green. We can hence use this indicator to locate this narrow pH range. In other words, in order to use the indicator effectively in this range, we should have a solution for which pH is very near to 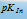 of the indicator. The colour change of an indicator can, thus, be summarised in the following tabular form,
of the indicator. The colour change of an indicator can, thus, be summarised in the following tabular form,
|
|
First change of colour
|
Mid point of change
|
Colour change complete
|
|
[H+]
|
10 KIn
|
KIn
|
0.1 KIn
|
|
pH
|
PKIn - 1
|
PKIn
|
PKIn + 1
|
It is for this reason that we make use different indicators for different systems.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Theories of Indicators questions? Theories of Indicators topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Theories of Indicators related problems. We provide step by step Theories of Indicators question's answers with 100% plagiarism free content. We prepare quality content and notes for Theories of Indicators topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours