Terminology of co-ordination compounds
(1) Central metal atom or ion : A complex ion contains a metal atom or ion known as the central metal atom or ion. It is at times also called as a nuclear atom.
(2) The complex ion : It is an electrically charged radical which is formed by combination of the simple cation with one or more neutral molecules or simple anions or in some of the cases positive groups also.
(3) Ligands : Neutral molecules or ions which attach to the central metal ion are called ligands. The donor atom related with the ligands supplies lone pair of electrons to central metal atom (forming the dative bond) may be one or two more. Monodentate (one donor atom), bidentate (two donor atom), tridentate (three donor atom) etc.
Monodentate Ligands (they have one donor site)
Table: Anionic Ligands (Negative legands)
|
Formula
|
Name
|
Formula
|
Name
|
|
X-
|
Halo
|
O22-
|
Peroxo
|
|
:OH-
|
Hydroxo
|
CH3COO-
|
Acetato
|
|
CN-
|
Cyano
|
NO3-
|
Nitrato
|
|
O2-
|
Oxo
|
S2O32-
|
Thiosulphato
|
|
NH2-
|
Amido
|
NO2-
|
Nitrito
|
|
S2-
|
Sulphido
|
CO32-
|
Carbonato
|
|
CNS-
|
Thiocyanato
|
SO42-
|
Sulphato
|
Table: Neutral Ligands
|
Formula
|
Name
|
Formula
|
Name
|
|
CO
|
Carbonyl
|
:NH3
|
Amminato
|
|
PH3
|
Phosphine
|
H2O
|
Aqua
|
|
NO
|
Nitrosyl
|
C2H5N:
|
Pyridine (py)
|
Table: Cationic Ligand (Positive)
|
Formula
|
Name
|
Formula
|
Name
|
|
NO2+
|
Nitronium
|
NO+
|
Nitrosonium
|
|
H2NNH3+
|
Hydrazinium
|
|
|
Polydentate ligands (with two or more donor site)
Table
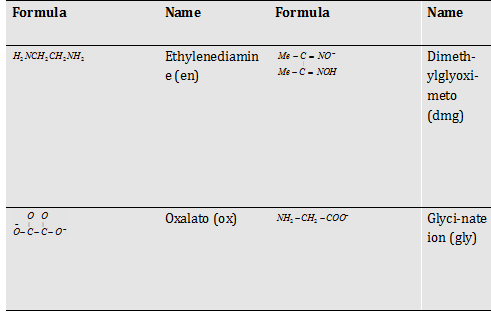
Table
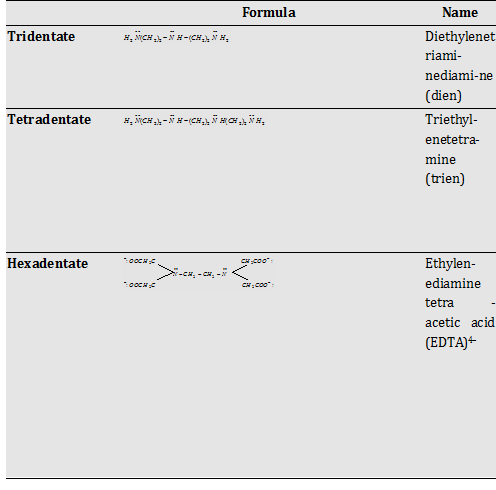
Chelating Ligand : The polydentate ligands when bind to the central metal ion they form a ring called as chelate and the ligand is referred as chelating ligand.
Ambidentate ligands : A ligand which possesses two donor atom but in forming complex it utilizes only one atom depending upon the condition and type of complex.
NO2 (nitro) , ONO (nitrito), CN (cyano), NC (isocyano), SCN (thiocyanide), NCS (isothiocyanide)
p- acid ligand : Ligands which are capable of accepting an appreciable amount of p- e- density from the metal atom into emptying Π or Π. orbital or their own called Π - acceptor or Π - acid ligands for example CO.
(4) Co-ordination Sphere : Ligand with central metal ion is kept in square bracket [ ] retains its identity in the same form is called co-ordination sphere (non-ionisable)
(5) Co-ordination Number : Number of monodentate ligands attached to central atom/ion are called coordination number of the central metal atom/ion.
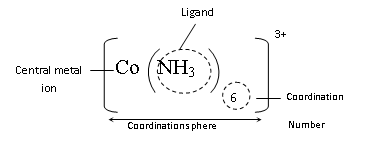
(6) Ionisation Sphere : The part present outside of the square bracket is called ionization sphere (ionisable).
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Terminology of co-ordination compounds questions? Terminology of co-ordination compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Terminology of co-ordination compounds related problems. We provide step by step Terminology of co-ordination compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for Terminology of co-ordination compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours