When two molecules approach each other, the charges in each are disturbed and redistributed in a fashion that the average distance between the unlike charges in the two molecules is a little smaller than that between the like charges. Thus, electrostatic attraction wins over the repulsion and gives rise to intermolecular attractive forces. Because of the fact that intermolecular forces are effective only over short distances, they are called short range forces. Moreover, these forces do not obey the inverse square law.
The maximum distance up to which a molecule can attract some other molecule is known as the range of the intermolecular force.
The intermolecular forces (F) of attraction can be defined as given:
1) Cohesion. The force of attraction between molecules of the same substance is called the force of cohesion. The tension of cohesion is maximumized in solids, lesser in liquids and least in gases. It is for this reason that the solids have definite shape and resist all deforming forces.
Illustration: The solids exhibit their rigid character and definite shape due to strong force of cohesion between their molecules.
2) Adhesion: The force of attraction between the molecules of the different substances is called the force of adhesion.
Illustration: (i) A piece of paper sticks to another due to large force of adhesion between the paper and the gum molecules.
(ii) Paint sticks to wood (and other surfaces) due to large force of adhesion between the surface of wood and paint.
It is found that when a liquid is free from the external forces, it always gets the shape of a spherical drop. It is because, for a required mass, a sphere has the least field area. It seems that the field of every liquid has usually a tendency to have least field area and in this respect, it functions like a stretched membrane having a force in all directions parallel to the surface. This force in the surface of a liquid is known as surface tension.
Thus, surface tension is that characteristics of a liquid by virtue of which, it functions like an elastic stretched membrane with a tendency to contract, so as to take a minimum surface area.
Surface tension can be described in given possible ways:
(i) The property of a liquid on account of which it tends to keep minimum number of molecules in its free surface is defined as surface tension.
(ii) The characteristics of a liquid, on account of which it leads to minimize it's free surface area, is known surface tension.
(iii) The work, needed to be done in increasing the free surface of a liquid by unity at constant temperature, is defined as surface tension.
(iv) The force operating per unit length of an imaginary line drawn on the free liquid surface at right angles to the line and in the plane of liquid surface is defined as surface tension.
Properties of surface Tension
|
|
- Depends only on the nature of the liquid.
- Unit of surface tension, N/m
- Dimension of surface tension, ML°T-2.
|
Phenomena due to surface tension
(i) Lead balls are spherical in shape.
(ii) Rain leaves and a globule of mercury placed on glass plate are spherical.
(iii) Hair of shaving painting brush, when goes in water spread out, but as soon as it is taken out. Its fur sticks together.
(iv) A greased needle saved gently on the free surface of water in a beaker does not sink.
(v) Similarly, insects may go on the free area of water without drowning.
(vi) Bits of computer gum go irregularly when occupied on water surface.
Molecular therory of surface tension
Before explaining surface tension on the basis of molecular theory, the following points may be noted:
(i) On the average, particles are divided by a distance of the order of 10-10 m and exert a force of attraction of the order of 10-11 N on each other.
(ii) The force of attachment between the molecules is due to electrical interaction between the charges, as the gravitational force between two molecules at a distance of 10-10 m is only of the order of 10-50 N.
(iii) Particles forces do not obey inverse square law.
(iv) The molecular forces are effective only over short distances. For that point, they are known as short-range forces.
(v) The maximum distance up to which a particle can attract some other molecules is known as the range of the intermolecular forces.
(vi) A sphere of radius same to the range of the molecular force about a given molecule as centre is called its sphere of influence.
(vii) A thin layer of liquid close its surface and having thickness equal to the molecular range for that liquid is called surface film.
It is the surface layer, which is responsible for the process of surface tension.
|
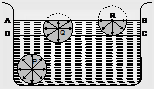
Explanation: In Fig. ABCD represents the surface layer of a liquid, where BC or AD is same to the particle range, suppose the three particles P, Q and R of a liquid. The circles show their spheres of influence.
|
|
The molecule P is well inside the liquid and is attracted equally in all directions by the other molecules lying within its sphere of influence. So there is no given cohesive tension on the molecule P in any position. In the part of the particle Q, more than half of its sphere of influence is above the liquid area. There are more particles performing it in downward direction than in upward direction. So it feels a total inward pull, which relays upon relative value of the molecules between the lower and the upper halves of its sphere of influence. The inward pull is maximized on the particles, such as the particle R relaying on the surface. This is because of the fact that the lower half of the sphere of influence of such a molecule is completely full of the liquid particle, while the upper is empty except for a few particles in the form of vapors.
Whenever it is required to move a molecule from the interior of the liquid to the surface area, a quantity of work has to be completed on it against the inward force of attraction. This work seems as the additional potential energy of the particles on the surface. Thus, the potential energy of the molecules in the surface film is greater than that of the molecules in the interior of the liquid. But in the kind of stable state equilibrium, a function has the minimum potential power. In order to retain minimum potential power and hence the stable state equilibrium, the surface layer goes to have the minimum surface area so that the number of molecules in the surface film may be minimum.
Thus, the free area of surface of a liquid at rest leads to have the minimum area and thereby, it behaves like a stretched membrance.
|
Surface tension is defined as the force per unit length in the plane of a liquid surface, operating at right angles on both sides of the imaginary line drawn on the surface
T = F /l
|
|
Email based Physics assignment help - homework help at Expertsmind
Are you searching physics expert for help with Surface tension questions? Surface tension topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Physics assignment help and physics homework help. Live tutors are available for 24x7 hours helping students in their Surface tension related problems. We provide step by step Surface tension question's answers with 100% plagiarism free content. We prepare quality content and notes for Surface tension topic under physics theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving physics queries in excels and word format.
- Best tutoring assistance 24x7 hours