Stereo isomerism or space isomerism : Here the isomers differ only in the spatial arrangement of atoms of groups about the central metal atom. It is generally of two types:
(i) Geometrical or Cis-trans isomerism : This isomerism arises due to the difference in geometrical arrangement of the ligands around the central atom. At the time when identical ligands occupy positions near to each other called cis-isomer. At the time when the identical ligands occupy positions opposite to each other called trans -isomer. It is very common in disubstituted complexes with co-ordination number of 4 and 6.
- Complexes of co-ordination number 4
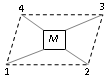
Tetrahedral geometry : In this case all the four ligands are symmetrically arranged with respect to one another as such geometrical isomerism is not possible.
Square planar geometry : The four ligands occupy position at the four corners and the metal atom or ion is at the center and lie in the same plane.
Type : I [Ma2b2], M=Pt, a= Cl, b=NH3
Example :[PtCl(NH3)(Py)2]

Complexes of the co-ordination number 6 Octahedral geometry : Here the metal atom or ion lies at the center and 1 to 6 position are occupied by the ligands.
Cis-Positions : 1-2, 2-3, 3-4, 4-5
Trans - position : 1-4, 2-5, 3-6
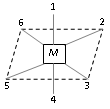
Type -I Ma4b2, M = Co, a = NH3, and b = Cl
Example : [CoCl2(NH3)4]+ ion
Type -II [Ma3b3], M = Rh, a = Cl and b = Py
Example : [RhCl3(Py)3]
Type -III [M(aa)2(en)2]++, 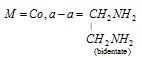
B = Cl (monodentate)
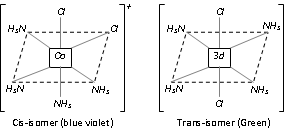
(ii) Optical isomerism
(a) Optical isomers are mirror images of each other and have chiral centers.
(b) The mirror images cannot be super imposed and do and have the plane of symmetry.
(c) The optical isomers have similar physical and chemical properties but differ in rotating the plane of plane polarized light.
(d) The isomer which rotates the plane polarized light to the right is called dextro rotatory (d-form) and the isomer which rotates the plane polarized light to the left is called laevorotatory (l-form)
Example : [Ma2b2c2]n-+ ; [Pt(py)2(NH3)2Cl2]2+
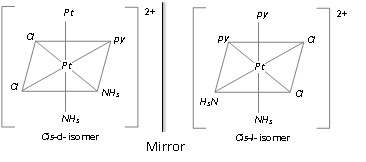
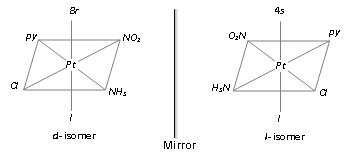
[M ab c d e f]; Pt(py)NH3NO2ClBr]
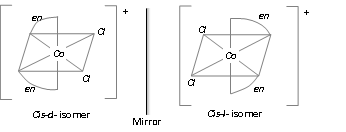
[M(AA)3]n+; [CO(en)3]3+
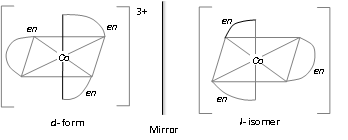
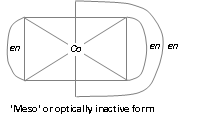
[M(AA)2a2]n+-, [Co(en)2Cl2]+
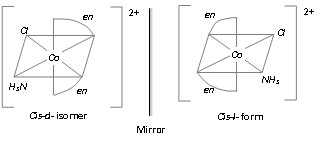
[M(AA)2ab]n+-, [Co(en)2NH3Cl]2+
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Stereo Isomerism questions? Stereo Isomerism topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Stereo Isomerism related problems. We provide step by step Stereo Isomerism question's answers with 100% plagiarism free content. We prepare quality content and notes for Stereo Isomerism topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours