When heat energy flows into an object, the temperature of the object generally rises.
The heat required raising the temperature of unit mass of a body through 1 oC or (1 oK) is called simply specific heat of the material of the object.
C = (1/m).(ΔQ/ΔT) ... (i)
The SI unit of specific heat is J/kg K. Heat is so commonly measured in calories; so the practical unit cal/g oC is also used quite rarely. The specific heat capacity of water is approximately 1 cal/g °C.
From Eq. (1), we can define the specific heat of a substance as "the amount of energy needed to raise the temperature of unit mass of that substance by 1°C (or 1 K)". A closely related quantity is the Molar specific heat C. It is defined as,
C = (1/n).(dQ/dT) ... (ii)
where n is the number of moles of the object. If M is the molecular mass of the object, then n = m/M were m is the mass of the substance and, C = (M/m).(dQ/dT) ... (iii)
The SI unit of C is J / mole K.
Key points:
(a) It depends on nature of material of object. Dulong and petit has found formula for elemental solids that (with few exceptions such as carbon)
Atomic weight ´ Specific heat = 6 cal / oC
So, heavier the element lesser will be the specific heat, i.e., CHg < CCu< CAl
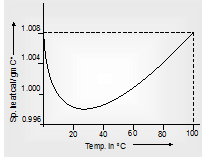
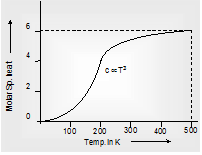
(b) Specific heat of a substance also depends on temperature (particularly at low temperatures) the variation of specific heat with temperature for water is shown in Fig (A) for metals in Fig (B). This temperature dependence of specific heat is usually neglected.
|
|
|
|
Fig. (A) Water
|
Fig. (B) Metals
|
(c) Specific heat also depends on the state of object, i.e., gas, liquid or solid e.g., specific energy of solid copper will be different from that of liquid copper.
|
Specific heat of
|
In cal/g °C
|
In SI units, i.e., J/kg K
|
|
Ice (solid)
|
0.5
|
2100
|
|
Water (liquid)
|
1
|
4200
|
|
Steam (gas)
|
0.47
|
1970
|
(d) If a substance is undergoing change of state which takes place at constant temperature (called isothermal change), specific heat, C = (1/m).(dQ/dT) = C = (1/m).(dQ/0) = ∞ [as ΔT = 0]
i.e., specific heat of a substance at its melting point or boiling point or isothermal change is infinite.
(e) Specific heat is found to be maximum for hydrogen (3.5 cal/gm °C) than for water (1 cal/gm °C = 4200 J/kg K). For all other object specific heat is lesser than 1 cal/gm °C and is minimum for radon and actinium (= 0.22 cal/gram °C)
(f) If the temperature of a object modifies without transfer of heat with the surroundings (adiabatic change) as in shaking a liquid or compressing a gas,
C= 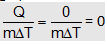 [as Q = 0]
[as Q = 0]
i.e., specific heat of a substance, when it undergoes adiabatic modification, is zero.
(g) Specific heat of a substance can also be minus. Negative specific heat denotes that in order to raise the temperature, some quantity of heat is to be withdrawn from the object. Specific heat of saturated water vapors is negative.
(h) When specific heats are calculated, the values contain are also found to depend on the conditions of the experiment. In simple calculations made at constant pressure are different from those at fixed volume. For liquids and solids this difference is very small and generally neglected. The specific heat of gases is quite different under constant pressure condition (cp) to constant volume condition (cv).
(i) As by definition c = (Q/m ΔT), heat required to change the temperature of m gm of a substance through ΔT:
Q = mc ΔT
and as ΔT = (Q/mc), greater the specific heat of a substance lesser will be the change in temperature for a given mass when same amount of heat is given. Now as specific heat of water is very large (1 cal/g °C), by releasing or observing large amount of heat its temperature modifies by small amounts. That is why; it is used in hot water bottles or as coolant in radiators. That is how the sea changes the climate of nearby coastal land.
Email based Physics assignment help - homework help at Expertsmind
Are you searching physics expert for help with Specific Heat questions? Specific Heat topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Physics assignment help and physics homework help. Live tutors are available for 24x7 hours helping students in their Specific Heat related problems. We provide step by step Specific Heat question's answers with 100% plagiarism free content. We prepare quality content and notes for Specific Heat topic under physics theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving physics queries in excels and word format.
- Best tutoring assistance 24x7 hours