Sodium
(1) The ores of sodium : NaCl(common salt), NaNO3 (chile salt petre), Na2SO4.10H2O (Glauber's salt), borax (sodium tetraborate or sodium borate, (Na2B4O7. 10 H2O).
(2) Extraction of sodium : It is manufactured by the electrolysis of fused sodium chloride in the presence of CaCl2 and KF using graphite anode and iron cathode. This procedure is called as Down process.
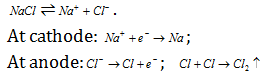
Sodium cannot be extracted from aqueous NaCl because 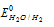 (-0.83V) is more than
(-0.83V) is more than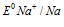 (-2.71V).
(-2.71V).
The anode and cathode are separated by the means of a wire gauze to prevent the reaction between Na and Cl2 .
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Sodium questions? Sodium topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Sodium related problems. We provide step by step Sodium question's answers with 100% plagiarism free content. We prepare quality content and notes for Sodium topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours