Silver:
(A) Ores:
- Argentite (silver glance) Ag2S,
- Horn silver (AgCl) Ruby silver (Pyrargyrite) 3Ag2S.Sb2S3.
(B) Extraction: Cyanide process or Mac Arthus-Forrest cyanide process: This process relies upon the fact that silver, the sulphide or chloride of it, makes soluble complex along with alkali cyanides in the silver. In the existence of blast of air this process indicates that the silver compounds will dissolve in solution of alkali cyanides.
The reaction below explained the process:
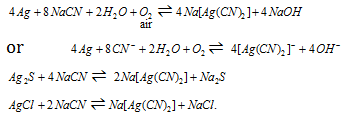
The reaction along with the sulphide is reversible and accumulation of Na2S has to be avoided. The excess of air is constantly passed via the solution that oxidizes Na2S into sulphate and thiosulphate.
The reaction of it is like this:
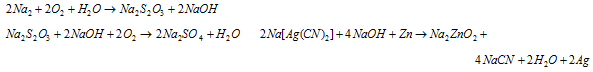
(C) Extraction of Ag from argentiferrous lead (PbS + Ag2S)- Parke's Process : It is based upon the following facts
a) Molten Zn and Pb are immiscible; the upper layer is formed by zinc
b) In molten Zn, Ag is much more soluble
c) Zn-Ag alloy solidifies previous than molten Pb
d) Zn being volatile can be separated from Ag through the distillation method. Ag can be purified by using cupellation.
Properties of Silver:
The properties of silver are as follow:
a) It is best conductor of heat and electricity.
b) Silver being soft and it is alloyed.
c) The formation of the silver alloy is expressed like its purity i.e. the quantity of Ag present in 1000 parts of the alloy Ag does not respond with dilute HCl or dilute H2SO4 and aqua regia although alloy reacts with dilute HNO3 and concentrated HNO3 anf creating NO and NO2 correspondingly.
d) Silver is the white lustrous metal
e) Chlorine as well reacts with Ag to make AgCl.
f) Silver alloy is employed for making jewellery consists of 80% Ag and 20% Cu.
2Ag + Cl2 → 2AgCl
Hot conc. H2SO4 reacts with Ag forming SO2 like Cu
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Silver questions? Silver topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Silver related problems. We provide step by step Silver question's answers with 100% plagiarism free content. We prepare quality content and notes for Silver topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours