Shape of orbitals
(1) Shape of 's' orbital
(i) For the 's' orbital l=0 & m=0 so 's' orbital have one unidirectional orientation only that is the probability of finding the electrons is same in all the directions.
(ii) The size and energy of the 's' orbital with the increasing 'n' will be given by 1s < 2s < 3s < 4s.
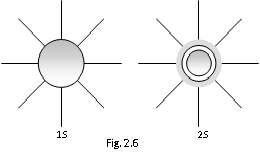
(iii) s-orbitals also called as radial node or modal surface. However there is no radial node for 1s orbital since it is beginning from the nucleus.
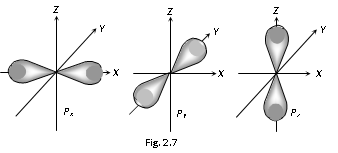
(2) Shape of 'p' orbitals
(i) For the 'p' orbital l=1, & m=+1,0,-1 means that there are three 'p' orbitals, which can be symbolised as px, py, pz
(ii) The shape of 'p' orbital is a dumb bell in which the two lobes on the opposite side are separated by the nodal plane.
(iii) p-orbital has the directional properties.
(3) Shape of 'd' orbital
(i) For 'd' orbital l =2 then values of 'm' can be given as -2, -1, 0, +1, +2. This shows that 'd' orbitals has five orbitals which are given by 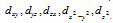
(ii) Each 'd' orbital is identical in size, shape and energy.
(iii) The shape of the d orbital is a double dumb bell.
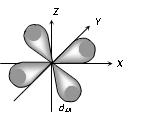
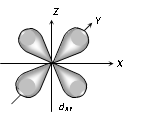
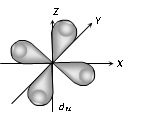
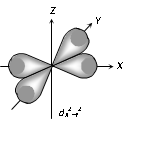
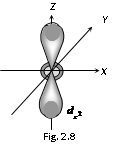
(iv) It also has directional properties.
(4) Shape of 'f' orbital
(i) For 'f' orbital the l=3 then the values of 'm' can be given as -3, -2, -1,0,+1,+2,+3. It shows that 'f' orbitals have seven orientation which can be given as 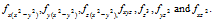
(ii) The 'f' orbital is quite complicated in shape.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Shape of orbitals questions? Shape of orbitals topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Shape of orbitals related problems. We provide step by step Shape of orbitals question's answers with 100% plagiarism free content. We prepare quality content and notes for Shape of orbitals topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours