Schroinger wave equation
(1) The Schrodinger wave equation was given by Erwin Schrödinger in the year 1926 and based on dual nature of an electron.
(2) In it electron it is described as a three dimensional wave in the electric field of the positively charged nucleus.
(3) The probability of getting the electron at any point around the nucleus can be determined by the help of Schrodinger wave equation which is given as follows,
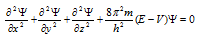
Here x, y and z are the 3 space co-ordinates,
m = mass of an electron,
h = Planck's constant,
E = Total energy,
V = potential energy of electron,
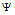 = the amplitude of wave also known as wave function,
= the amplitude of wave also known as wave function,
∂ = for an infinitesimal change.
(4) The Schrodinger equation of the wave can also be written as,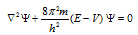
Here 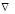 = laplacian operator.
= laplacian operator.
(5) Physical significance of 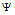 and
and 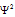
(i) The wave function 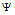 represents amplitude of the electron wave. The amplitude
represents amplitude of the electron wave. The amplitude 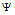 is therefore a function of space co-ordinates and time that is
is therefore a function of space co-ordinates and time that is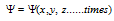
(ii) For the single particle, a square of the wave function 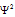 at any point of time is proportional to the probability of finding the particle at that point.
at any point of time is proportional to the probability of finding the particle at that point.
(iii) If 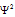 is maximum than probability of finding e- is maximum around nucleus and the place where probability of finding e- is maximum is called electron cloud electron density, or an atomic orbital. It is not similar from the Bohr's orbit.
is maximum than probability of finding e- is maximum around nucleus and the place where probability of finding e- is maximum is called electron cloud electron density, or an atomic orbital. It is not similar from the Bohr's orbit.
(iv) The solution of this equation provides us a set of number known as quantum numbers which describe the specific or definite energy state of the electron in an atom and information about the shapes and orientations of the most possible distribution of the electrons around the nucleus.
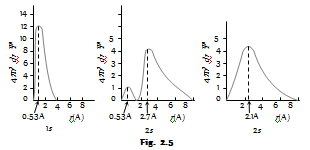
Radial probability distribution curves : The radial probability can be given as 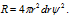
The plats of R distance from the nucleus are given as follows
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Schrodinger wave equation questions? Schrodinger wave equation topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Schrodinger wave equation related problems. We provide step by step Schrodinger wave equation question's answers with 100% plagiarism free content. We prepare quality content and notes for Schrodinger wave equation topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours