Salt hydrolysis
It is reaction of the cation or the anion or both the ions of the salt with water to produce the acidic or basic solution. Hydrolysis is reverse of neutralization.

(1) Hydrolysis constant : The general equation for the hydrolysis of a salt (let it be BA), 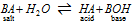
Applying the law of chemical equilibrium, we can get
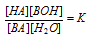 , here K is equilibrium constant.
, here K is equilibrium constant.
Since water is present in very large excess in the aqueous solution, its concentration [H2O] may be regarded as constant so,
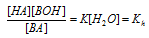
where Kh is called the hydrolysis constant.
(2) Degree of hydrolysis : It is defined as the fraction (or percentage) of the total salt which is hydrolysed at the equilibrium. For instance, if 90% of a salt solution is hydrolysed, it's the degree of hydrolysis and is 0.90% or as 90%. It is generally represented by h.
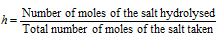
|
Types of salt
|
Exp. for Kh
|
Exp. for h
|
Exp. for pH
|
|
(i) Salt of weak acid and strong base
|
Kh=Kw / Ka
|
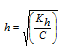
|
pH=- [log Kw+log Ka- log C]
|
|
(ii) Salt of strong acid and weak base
|
Kh=Kw / Kb
|
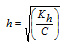
|
pH=- [log Kw- log Kb+ log C]
|
|
(iii) Salt of weak acid and weak base
|
|
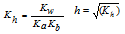
|
pH=- [log Ka+ log Kw- log Kb]
|
(iv) Salts of the strong acids and strong bases do not undergo hydrolysis (they undergo only the ionization process) hence the resulting aqueous solution is neutral in nature.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Salt hydrolysis questions? Salt hydrolysis topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Salt hydrolysis related problems. We provide step by step Salt hydrolysis question's answers with 100% plagiarism free content. We prepare quality content and notes for Salt hydrolysis topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours