Rules for filling of electrons in various orbitals
The atom is built up by filling the electrons in several orbitals according to the rules stated as follows,
(1) Aufbau's principle
The principle states that electrons are added one by one to the various orbitals in the order of their increasing energy beginning with the orbital of lowest energy. The increasing order of the energy of various orbitals is
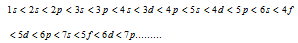
(2) (n+l) Rule
In the neutral isolated atom, lower the value of (n + l) for the orbital, lower is its energy. Though, if the two different types of the orbitals have the same value of (n + l), the orbitals with the lower value of n has lower energy.
(3) Pauli's exclusion principle
In accordance to this principle "no two electrons in the atom will have same value of all four quantum numbers".
If one of the electron in an atom has the quantum numbers n=1, l=0, m=0 and s=+1/2, then no other electron can have the same four quantum numbers. Or we can say that, we cannot place two electrons with same value of s in a 1s orbital.
The orbital diagram 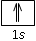 does not represent the possible arrangement of the electrons. Because there are two possible values only of s an orbital can hold not more than the two electrons.
does not represent the possible arrangement of the electrons. Because there are two possible values only of s an orbital can hold not more than the two electrons.
(4) The Hund's Rule of the maximum multiplicity
This rule deals with filling of the electrons in an orbitals having equal energy (degenerate orbitals). This rule states that the,
"The Electron pairing in the p, d and f orbitals cannot occur until each of the orbitals of a given subshell contains one electron in each or is singly occupied".
This is because of the fact that the electrons being identical in charge, repel each other when are present in the same orbital. The repulsion can however be minimized if the two electrons move as far as possible by occupying the different degenerate orbitals. All the unpaired electrons in the degenerate set of orbitals will have the same spin.
As we now know that the Hund's rule, allows us see how the three electrons are arranged in the p orbitals.
An important point to be kept in mind is that all singly occupied orbitals should have electrons with the parallel spins that is in the same direction either-clockwise or anticlockwise.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Rules for filling of electrons questions? Rules for filling of electrons topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Rules for filling of electrons related problems. We provide step by step Rules for filling of electrons question's answers with 100% plagiarism free content. We prepare quality content and notes for Rules for filling of electrons topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours